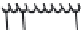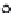Biomedical Engineering Reference
In-Depth Information
Normal
Alzheimer's
Neuroibrillary
tangles
Neuron
Amyloid
plaques
Plaque
Tangle
(A)
(B)
FIGURE 16.1
Amyloid plaques and neuroi brillary tangles. (A, Reprinted courtesy of Alzheimer's dis-
ease Research, a program of the American Health Assistance Foundation. www.ahaf.org/alzheimers/.; B,
Reprinted courtesy of Science Foto Library.)
NH
2
Neurotoxicity
Extracellular
Fibrillogenesis
APPsβ
APPsα
APP
Aβ
40/42
p3
α-Secretase
β-Secretase
γ-Secretase
γ-Secretase
α-Stub
β-Stub
Intracellular
COOH
FIGURE 16.2
The amyloidogenic pathway.
precursor protein (APP), a transmembrane protein of unknown function. The APP is cleaved in
two different regions of its extracellular amino-terminal domain by the enzymes a-secretase and
b-secretase, and subsequently the remaining transmembrane protein sections, the a- and b-stubs,
are cleaved by g-secretase, giving rise to the peptides p3 and Ab
40/42
, respectively (Figure 16.2). In
particular, the Ab
42
peptide undergoes oligomerization and deposition, leading to microglial and
astrocytic activations, oxidative stress, and progressive synaptic injury.
The neuroi brillary tangles are bundles of paired helical i laments constituted mainly by the tau
protein, a widely expressed protein from the microtubule-associated family. Under normal condi-
tions, tau maintains microtubule stability inside the cell but in AD the protein exists in a phos-
phorylated form, which aggregates into tangled clumps. The formation of the tangles reduces the
number of tau proteins that are able to bind and stabilize the microtubules, which thus disintegrate,
ultimately leading to cytoskeletal degeneration and neuronal death.
The complex and multifactorial pathogenesis of AD is not fully understood, and throughout the
years several theories have been formed to explain the molecular mechanisms underlying the dis-
ease. In the 1970-1980s, the “cholinergic hypothesis” for AD was formulated based on the observed
dramatic loss of cholinergic markers, such as choline acetyltransferase and acetylcholinesterase, in