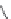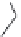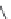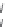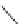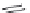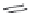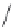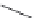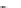Biomedical Engineering Reference
In-Depth Information
desired pharmacological effect typically resides in one enantiomer, whereas the other stereoisomer(s)
are pharmacologically inactive or possess different pharmacological effects. Thus, chiral drugs should
preferentially be resolved into stereochemically pure isomers prior to pharmacological examination.
Since many, especially of older date, synthetically prepared chiral biologically active compounds have
been described pharmacologically as racemates, much of the pharmacological literature should be
read and interpreted with great care.
Figure I.6 exemplii es the importance of stereochemistry in studies of the relationship between
structure and pharmacological activity (SAR studies).
The upper part of Figure I.6 shows the four stereoisomers, which actually are two pairs of
enantiomers of two diastereomeric compounds. These 1-piperazino-3-phenylindans were synthe-
sized, resolved, structurally analyzed, and pharmacologically characterized as part of a compre-
hensive drug research program in the i eld of central biogenic amine neurotransmission. Whereas
one of these stereoisomers turned out to be inactive, two of them were inhibitors of dopamine (DA)
and noradrenaline (NE) uptake, and one isomer showed antagonist effects at DA, NE, and serotonin
(5-HT) receptors. It is evident that a pharmacological characterization of a synthetic mixture of
these compounds would be meaningless.
The 3-isoxazolol amino acid, APPA, is an analogue of the standard agonist, AMPA, for the
AMPA subgroup of excitatory glutamate receptors (Chapter 15). APPA was tested pharmacologi-
cally as the racemate, which showed the characteristics of a partial agonist at AMPA receptors.
Subsequent pharmacological characterization of the pure enantiomers quite surprisingly disclosed
that (
S
)-APPA is a full AMPA receptor agonist, whereas (
R
)-APPA turned out to be an AMPA
antagonist. This observation prompted intensive pharmacological studies, and as a result it was
demonstrated that administration of a i xed ratio of an agonist and a competitive antagonist always
provides a partial agonist response at an efi cacy level dependent on the administered ratio of com-
pounds and their relative potencies as agonist and antagonist. This phenomenon was named “func-
tional partial agonism.” An interesting aspect of this pharmacological concept is that administration
of an antagonist drug inherently establishes functional partial agonism together with the endog-
enous agonist at the target receptor.
R
R
R
R
N
N
N
N
N
N
N
N
X
(
R
)
X
(
S
)
X
(
R
)
X
(
S
)
(
R
)
(
S
)
(
R
)
(
S
)
Y
(1
R
, 3
S
)-Enantiomer
Y
Y
Y
(1
S
, 3
R
)-Enantiomer
(1
R
, 3
R
)-Enantiomer
(1
S
, 3
S
)-Enantiomer
DA-/NE-/5-HT-antagonist
DA-/NE-uptake inhibitors
Inactive
O
O
OH
OH
(
S
)
(
R
)
HO
HO
N
N
H
2
N
H
2
N
O
O
Ph
Ph
(2
S
)-APPA
(2
R
)-APPA
FIGURE I.6
Chemical structures of the four stereoisomers of 1-piperazino-3-phenylindans and the two
enantiomers of the phenyl analogue of AMPA (APPA).