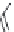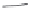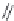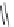Biomedical Engineering Reference
In-Depth Information
O
O
HO
OH
O
HN
O
O
HO
OH
HO
O
OH
H
2
N
HN
N
O
HO
KA (
15.83
)
Domoic acid (
15.84
)
(
S
)-ATPA (
15.85
)
O
HO
O
OH
O
HO
H
2
N
O
O
NH
N
HO
H
2
N
OH
O
H
2
N
I
(
S
)-5-I-Willardiine (
15.86
)
O
(2
S
,4
R
)-Me-Glu (
15.87
)
LY339434 (
15.88
)
O
OH
O
H
N
N
N
HO
O
HN
OH
O
O
2
N
H
N
O
OH
Cl
Cl
NS 102 (
15.89
)
LY382884 (
15.90
)
(
15.91
)
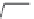
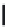
FIGURE 15.19
Structures of KA (
15.83
) and some KA receptor agonists (
15.84
-
15.88
), two competitive
(
15.89
and
15.90
) and one noncompetitive antagonist (
15.91
).
15.8 METABOTROPIC GLUTAMATE RECEPTOR LIGANDS
The cloning of the mGluRs and the evidence, which has subsequently emerged on their potential
utility as drug targets in a variety of neurological disorders, have encouraged medicinal chemists
to design ligands targeted at the mGluRs. In analogy to the iGluRs, several x-ray structures of a
mGluR ligand-binding construct (see Figure 12.7) including different ligands have been obtained
and afforded important structural knowledge of value, e.g., in the design of ligands.
15.8.1 M
ETABOTROPIC
G
LUTAMATE
R
ECEPTOR
A
GONISTS
The i rst agonist to show selectivity for mGluRs over iGluRs was (1
S
,3
R
)-ACPD (
15.92
) which has been
used extensively as a template for the design of new mGluR ligands. Introduction of a nitrogen atom in
the C4 position of
15.92
gave (2
R
,4
R
)-APDC (
15.93
) which displays an increased potency for group II
receptors compared to the parent compound while losing afi nity for group I and III receptors.
LY35 474 0 (
15.94
) displays low nanomolar agonist potency at mGluR2 and mGluR3, low micromo-
lar agonist potency at mGluR6 and mGluR8, while showing no activity at the remaining mGluRs.
ABHxD-I (
15.95
) displays potent agonist activity, comparable to Glu, at all three mGlu groups.
This observation has been of key importance in developing early models of the mGluR-binding
site. Compound
15.95
is quite a rigid molecule, which adopts a conformation corresponding to an
extended conformation of Glu. The observation that the compound is a potent agonist for all three
mGluR groups led the suggestion that Glu adopts the same extended conformation at all three recep-
tor groups, and that group selectivity is thus not a consequence of different conformations but rather
a consequence of other factors such as, steric hindrance.