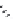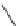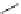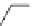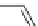Biomedical Engineering Reference
In-Depth Information
OH
OH
P
OH
H
2
N
H
2
N
O
O
HO
O
P
H
2
N
H
Cl
Cl
(
R
)-Baclofen (
15.35
)
CGP27492 (
15.36
)
Phaclofen (
15.37
)
OH
O
S
Cl
HO
H
2
N
O
O
P
N
Cl
OH
Cl
Saclofen (
15.38
)
CGP-55845 (
15.39
)
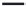
FIGURE 15.9
Structures of ligands for the GABA
B
receptor.
addiction are potential targets for GABA
B
agonist therapy. However, the use of GABA
B
agonists has
been limited due to serious side effects such as sedation, tolerance, and muscle weakness following
systemic administration.
15.5.5 L
IGANDS
D
IFFERENTIATING
THE
GABA
A
AND
GABA
C
R
ECEPTORS
IAA (
15.31
) is a naturally occurring metabolite of histamine. The compound has various neurologi-
cal effects, believed to be mediated by the central GABA
A
receptors. It penetrates the blood-brain
barriers on systemic administration and is therefore advantageous from a bioavailability perspective
compared to the other known standard ligands for the ionotropic GABA receptors.
Like other GABA analogs, IAA displays activities on the GABA
A
as well as on the GABA
C
receptors, being a partial agonist of both groups of receptors. In an attempt to deduce the structural
determinants for the activity of the respective receptor groups, a series of IAA analogs have been
synthesized.
The introduction of even small substituents in the 2-position of IAA was found to have detri-
mental effects on the activities of both receptor classes, suggesting that there is little space in the
orthosteric sites around this position in the IAA molecule (Figure 15.10). In contrast to the lack of
activity in the 2-substituted IAA analogs (
15.42
), several of the 5-substituted IAA analogs,
15.40
and
15.41
, retained the agonist properties at
ρ
1
GABA
C
receptors while exhibiting no activity at the
α
1
β
2
γ
2S
GABA
A
receptors (Figure 15.10).
The 5-Me-IAA analog (
15.40
) was docked into receptor-models of the GABA
A
α
1
β
2
interface and
into the orthosteric site on
ρ
1
GABA
C
receptors based on the bioactive conformations of the ligand
deduced from the previously mentioned pharmacophore model (see Section 15.5.2). The resulting
ligand orientation and receptor interactions are show in Figure 15.11. According to the models, the
main difference in the vicinity of the ligands in the orthosteric sites of the
α
1
β
2
γ
2
and the
ρ
1
receptors
is a threonine residue (Thr129) in the
α
1
subunit and a serine (Ser168) residue in the equivalent position
in
ρ
1
receptor makes it possible for the orthosteric site
to accommodate substituents in the 5-position of IAA. A mutagenesis study based on the above men-
tioned ligand-receptor docking experiments verii ed the Thr129 residue in the
ρ
1
. The smaller size of the Ser168 residue in the
α
1
subunit of the
α
1
β
2
γ
2
GABA
A
receptor and the corresponding Ser168 residue in
ρ
1
receptor as major molecular determinants
for the observed differences in agonist potencies between the two receptors.