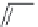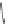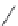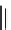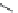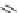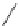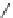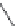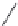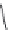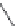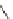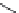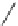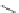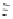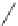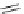Biomedical Engineering Reference
In-Depth Information
O
Cl
OH
H
N
N
NN
O
Cl
H
O
4
Fentanyl
U50,488
Tetrahydrocannabinol (THC)
OH
OH
O
N
N
NH
NH
N
5
CP55,940
Cytisine
Varenicline
HO
OH
OH
HN
H
2
N
N
N
O
O
Muscimol
THIP (Gaboxadol)
O
O
O
O
O
OH
OH
OH
HO
O
Trp-Pro-Arg-Pro-Gln-Ile-Pro
H
N
N
HS
N
O
O
Teprotide
N
-Succinylproline
Captopril
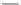
FIGURE I.4
Chemical structures of fentanyl, U50,488, tetrahydrocannabinol (THC), CP55,940, cytisine,
varenicline, muscimol, THIP (gaboxadol), teprotide,
N
-succinylproline, and captopril.
The main psychoactive constituent of
Cannabis sativa
, the highly lipophilic tetrahydrocannabi-
nol (THC) has been a useful tool for the identii cation of the two cannabinoid receptors, CB
1
- and
CB
2
-receptor operated by endocannabinoids. Since different preparations of
C. sativa
have psycho-
active effects, health authorities have been reluctant to accept THC and analogues as therapeutic
agents for the treatment of pain and other disease-related conditions. This may change with time,
as medicinal chemists have synthesized a number of cannabinoid receptor ligands, including the
receptor agonist CP55,940, which is markedly less lipophilic than THC (Chapter 19).
The nicotine acetylcholine receptors (nAChRs) have become key targets for therapeutic
approaches to treat pain, cognition disorders, depression, schizophrenia, and nicotine dependence.
For several reasons, nicotine has limited utility as a therapeutic agent, and a wide variety of nAChR
agonists have been synthesized and characterized (Chapter 16). (-)-Cytisine is a naturally occurring
toxin acting as a powerful nAChR agonist. Using (-)-cytisine as a lead structure, varenicline was
developed as a partial nAChR agonist showing an optimally balanced agonist/antagonist proi le for
smoking cessation.
Muscimol is another example of a naturally occurring toxin, which has been extensively used as
a lead for the design of specii c GABA receptor agonists and GABA uptake inhibitors (Chapter 15).
Muscimol, which is a 3-isoxazolol bioisostere of GABA, is a constituent of the mushroom
Amanita
muscaria
. Muscimol is toxic, it is metabolically unstable, and it interacts with the different GABA
synaptic mechanisms and with a broad range of GABA
A
receptor subtypes. The cyclic analogue of
muscimol, THIP (Gaboxadol) is highly selective for the therapeutically interesting extrasynaptic