Biomedical Engineering Reference
In-Depth Information
obesity and epilepsy as well as drugs of abuse such as cocaine, amphetamine, and “ecstasy.” Of
interest, high-resolution crystal structures have recently become available of bacterial homologues
of SLC6 transporters opening up entirely new possibilities for understanding how these trans-
porters operate at a molecular level and how their function can be altered by different types of
drugs.
14.2 NEUROTRANSMITTER TRANSPORTERS
BELONGING TO THE SLC6 FAMILY
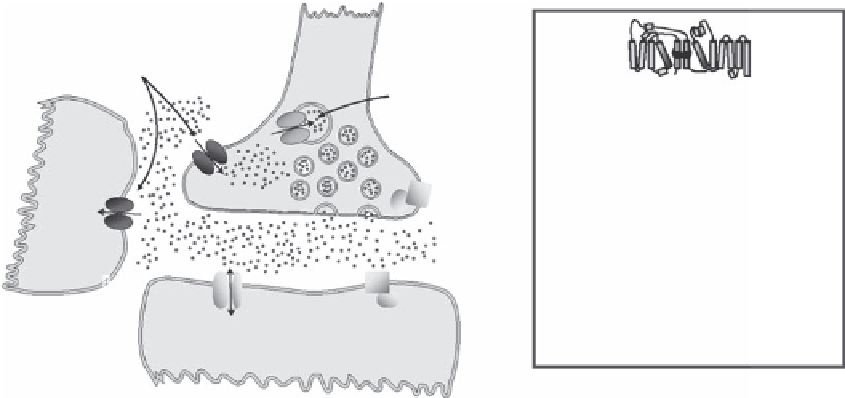
The availability in the synaptic cleft of the neurotransmitters dopamine, serotonin, norepinephrine,
glycine, and
-amino butyric acid (GABA) is tightly regulated by specii c transmembrane transport
proteins belonging to the SLC6 family (Figure 14.1 and Table 14.1). The transport proteins are
situated either in the presynaptic membrane or on the surface of adjacent glial cells, where they
mediate the rapid removal of the released neurotransmitters and thereby terminate their effect at the
pre- and postsynaptic neurons. Inside the presynaptic nerve endings, specii c vesicular transport-
ers sequester the neurotransmitters into vesicles, making them ready for subsequent release into
the synaptic cleft upon the arrival of the next stimulus. The plasma membrane neurotransmitter
transporters serve three main purposes: i rst, the transport proteins increase the rate by which the
released neurotransmitters are cleared from the synaptic cleft. This rapid removal of the released
neurotransmitters allows for 100-fold faster termination of neurotransmission than is possible with
simple diffusion. Second, reuptake may prevent diffusion of the neurotransmitters away from the
γ
Plasma membrane
neurotransmitter
transporters
(SLC6 family)
Vesicular
neurotransmitter
transporters
(SLC18 and 32)
Axon
SLC6
gene family members:
• Dopamine transporter (DAT)
• Serotonin transporter (SERT)
• Norepinephrine transporter (NET)
• GABA transporters (GAT-1, GAT-2, GAT-3)
• Glycine transporters (Glyt-1, Glyt-2)
• Betaine transporter (BGT1)
• Taurine transporter (TAUT)
• Creatine transporters (CT1 and CT2)
• Proline transporter (PROT)
• Cationic amino acid transporter (ATB[0+])
• Large neutral amino acid transporter (SBAT1)
• Neutral amino acid transporter (B
0
AT 1 )
• Imino acid transporter (SIT1)
• Three orphan transporters
• Prokarytotic homologues in bacteria and
archaea (>200 diferent, e.g., LeuT from
Aquifex aeolicus
)
Glial cell
Dopamine
5-HT
Norepinephrine
GABA
Glycine
R
R
G
GPCR
Na
+
, K
+
, Ca
2+
, Cl
-
Ligand-gated ion channel
FIGURE 14.1
The role of neurotransmitter transporters in synaptic signaling. Neurotransmitters are seques-
tered into synaptic vesicles through vesicular monoamine transporters (VMAT1-2) belonging to the SLC18
gene family or through vesicular inhibitory amino acid transporters (VIAAT) belonging to the SLC32 gene
family. Upon arrival of an axon potential, the synaptic vesicle releases its content of the neurotransmitter into
the synaptic cleft by fusion of the vesicle with the plasma membrane. The neurotransmitter exerts its effects
by activating ionotropic receptors, (ligand-gated ion channels), such as GABA
A
receptors, glycine receptors,
and 5-HT
3
receptors or via G-protein-coupled receptors (GPCRs) such as dopamine receptors, adrenocep-
tors, 5-HT receptors, and metabotropic GABA
B
receptors. The fast removal of the neurotransmitter from the
synaptic cleft is governed by the neurotransmitter transporter belonging to the SLC6 family located on the
presynaptic neuron (DAT, SERT, NET, GlyT2, GAT-1, and GAT-2) or on glia cells (GlyT-1, GAT-1, GAT-2, and
GAT-3). The neurotransmitter taken up by the presynaptic neuron allows recycling with a presumed savings
in synthetic cost.