Biomedical Engineering Reference
In-Depth Information
(A)
Depolarization
Repolarization
Closed
Open
Inactivated
(B)
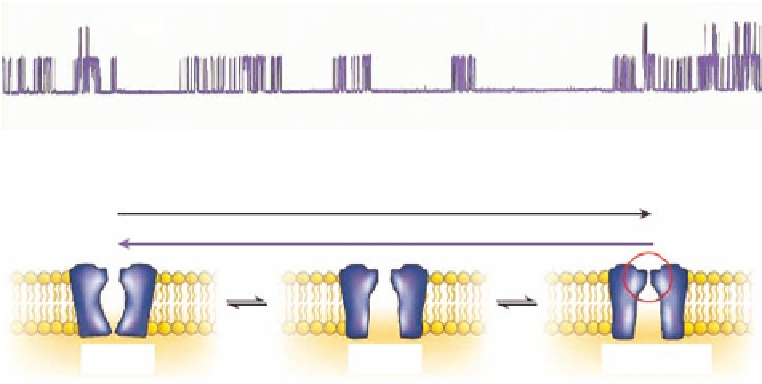
FIGURE 13.2
(A) Single channel recording of BK-type potassium channel. The baseline shown the closed
state and the upward del ections are opening of single channels. The current through the channels is about 20 pA.
(B) Opening, closing, and inactivation of ion channel. (Modii ed from Sanguinetti, M.C. and Tristani-Firouzi,
M.,
Nature
, 440, 463, 2006.)
Patch-clamp studies of single ion channels have shown that the duration of channel opening
ranges from a few
s to several hundred ms when exposed to a ligand or a voltage change (Figure 13.2).
In the absence of stimulus they either open less frequently or stay closed. The opening and closing
of ion channels is called gating, and at the single channel level it is described by the distribution
of open- and closed-times. Currents through all ion channels in the cell membrane can also be
measured by ripping a hole in the cell, which makes it possible to voltage-clamp the whole cell.
This whole-cell current depicts the sum of hundreds of ion channels, and the kinetics of the current
rel ect the average open- or closed-times of the channels.
The gating is a dynamic process rel ecting structural changes in the channel protein. The
opening of a channel is preceded by conformational changes, and to the extent that these changes
result in movement of charged segments of the molecule (voltage sensors), it can be followed by
measurement of small gating currents. Once the channel goes into the open state, the electri-
cal current carried by ions through the channel can be recorded with a resolution of about 1 pA
(10
−12
A). The activation of single channels is a discrete event, and as seen from the recordings
in Figure 13.2 the ion channels are either fully closed or fully open. Thus, it is possible to fol-
low the movements between the two conformational states with an amazing time- and current-
resolution.
The various types of ion channels gate differently: some channels open only transiently whereas
others stay open as long as the stimulus exists. Stimuli for ion channel activation are either (1) a
change in the membrane potential, (2) a change in the concentration of extracellular ligands (neu-
rotransmitters), (3) a change in the concentration of intracellular ligands (Ca
2+
, H
+
, cyclic nucleotides,
or G-protein subunits), or (4) mechanical stimulation (e.g., stretch). Once the channels are exposed
to the electrical or chemical stimuli they open or activate, and when the stimulus is removed, the
channels close in an opposite process called deactivation. A number of channel types do however
also close in the presence of the stimulus. It is a general physiological phenomenon that continued
stimulation of a signal process results in a decreasing output. This functional closure of ion channels
in the presence of stimulus is called inactivation and can occur either by parts of the channel protein
plugging the open pore after a short delay, by collapse of the pore, or by decreased coupling between
ligand-binding- and pore-domains (Figure 13.2B).
μ