Biomedical Engineering Reference
In-Depth Information
K
+
Na
+
Ca
2+
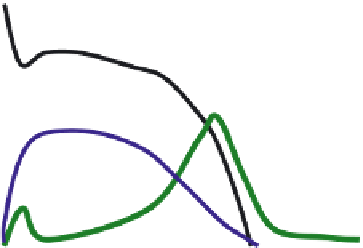
FIGURE 13.1
Cardiac action potential and time-course of selective Na
+
, Ca
2+
, and K
+
currents.
These two forces are generated by the concentration gradient and by the electrical i eld generated
by the membrane potential, respectively. The ion movement will stop once the two forces equal each
other, which happens at the so-called equilibrium potential. This potential is determined by the ion
distribution across the membrane, and for the typical intra- and extracellular ion concentrations the
equilibrium potentials are shown in Table 13.1.
The effects on the membrane potential of activation on selective ion channels are shown in
Figure 13.1. The cardiac action potential (AP) is initialized by opening of voltage-gated Na
+
chan-
nels, and the inl ux of the positively charged Na
2
+
ions leads to a fast positive shift in the membrane
potential (depolarization). Subsequently voltage-gated Ca
2+
channels are opened and the inl ux of
Ca
2+
ions keeps the membrane potential depolarized. Fast K
+
channels are activated early in the
response and attenuate the depolarization, but the key role of the K
+
currents is to terminate the
AP after about 350 ms when numerous K
+
channels open and the outl ow of the positively charged
K
+
ions mediate the repolarization.
The consequence of the sequential opening of Na
+
-, Ca
2+
-, and K
+
-selective channels, is thus
that the cell membrane potential will be pulled in the direction of the equilibrium potential for
these ion species, i.e., about +70 mV for Na
+
, +120 mV for Ca
2+
, and −93 mV for K
+
(Table 13.1).
Often the cell does not fully reach the equilibrium potential as shown for the cardiac action poten-
tial, since several types of channels are usually open at the same time. Likewise the impact on the
membrane potential of physiological or pharmacological ion channel block or activation depends
on the presence of other simultaneous conductances and is not just linearly correlated to the num-
ber of ion channels being affected. Thus, it can be complicated to predict the functional effect of
modulating ion channel function, and extensive target validation studies have to be conducted to
establish the anticipated role of an ion channel subtype in an organ.
13.1.3 G
ATING
OF
I
ON
C
HANNELS
While it was clear to Hodgkin and Huxley that a sequential increase in Na
+
and K
+
membrane
conductances underlies the neuronal action potential, their method could not reveal the nature of
the conductance pathway. This had to await another technological breakthrough. In 1976, Neher
and Sakmann reported the opening and closing of single acetylcholine-gated ion channels in stri-
ated muscle using a method by which they electrically isolated a patch of membrane
in situ
with
a glass pipette. The method was called patch-clamp with reference to the patch of tissue and the
clamp of the transmembrane (TM) voltage used to generate the electrical driving force. Since then
the method has been extensively used to describe the characteristics and function of ion channels
in all cells. Initially endogenous currents in cells were measured, but following the cloning area the
combination of this functional method and heterologous expression of cloned ion channels has been
a strong combination in the target-driven drug discovery process.