Biomedical Engineering Reference
In-Depth Information
Albeit the tyrosine kinase receptors share the overall activation mechanism, the family has
turned out to be rather heterogeneous with respect to the structure and ligand-receptor interaction.
Some of the receptors exist as monomers (e.g., the EGF receptor family) in the absence of agonist
whereas others exist as covalently linked dimers (e.g., the insulin receptor family) or noncovalently
linked dimers (e.g., the EPO receptor). In case of the monomers, agonist binding to either one or
both subunits will bring the two receptor subunits together, and thereby initiate the autophosphory-
lation. In case of the preformed inactive dimers, agonist binding will cause a conformational change
in the receptor, which brings the two intracellular kinases together and thus initiate the autophos-
phorylation. One of the best understood examples in this regard is the EPO receptor of which the
3D structure of the extracellular agonist-binding domain has been determined in the absence and in
the presence of EPO (Figure 12.9). In the absence of EPO the domain is a dimer in which the ends
are too far apart for the JAKs to reach each other. EPO binds to the same amino acids on the recep-
tor that forms the dimer interface and thereby tilts the two receptor subunits. This brings the JAKs
close together and initiate the autophosphorylation (Figure 12.9).
12.2.4 N
UCLEAR
R
ECEPTORS
Nuclear receptors are cellular proteins and are thus not embedded in the cell membrane like the
previously described receptors. In contrast to the membrane bound receptors, they bind small lipo-
philic compounds and function as ligand-modulated transcription factors. The nuclear receptors
have been classii ed according to the type of hormone they bind. Thereby, receptors have been
divided into those which bind steroids (glucocorticoids, progestestins, mineralocorticoid androgens,
and estrogens) and steroid derivatives (vitamin D
3
), nonsteroids (e.g., thyroid hormone, retinoids,
and prostaglandines), and orphan receptors for which the physiological agonist has yet to be discov-
ered. The receptor family is relatively small (~50 subtypes) of which 50% still belongs to the group
of orphan receptors.
D1
D1
EPO
D1
D1
D2
D2
D2
D2
34Å
73Å
JAK-2
JAK-2
JAK-2
JAK-2
P
P
P
P
P
P
(A)
(B)
(C)
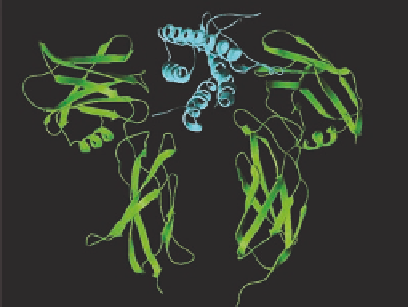
FIGURE 12.9
Cartoon of the activation mechanism of the erythropoietin (EPO) receptor, which belong to
the JAK/STAT receptor class of the superfamily of tyrosine kinase receptors. (A) The receptor is dimerized
in the inactive conformation by interaction of amino acids, which are similar to those involved in binding of
EPO and the intracellular JAKs are kept too far apart to initiate autophosphorylation. (B) Binding of EPO to
the dimer interface tilts the structure and brings the JAKs in close proximity, which initiates the autophospho-
rylation. (C) The actual structure of EPO (in cyan) bound to the extracellular receptor domains of the EPO
receptor (in green). The structure was generated using the program “Swiss PDB viewer 3.5” with coordinates
from Brookhaven Protein Data Base. (Adapted from Wilson, K.S. et al.,
Curr. Opin. Struc. Biol
. 9, 696, 1999.
With permission.)