Biomedical Engineering Reference
In-Depth Information
selective and l exible pharmacological manipulation of the receptor complex concerned. While
some receptors are associated with ion channels, others are coupled to second messenger systems.
The key steps in such enzyme-regulated multistep intracellular systems (Figure 12.2), also includ-
ing regulation of gene transcription by second messengers, represent novel targets for therapeutic
interventions.
12.2 RECEPTOR STRUCTURE AND FUNCTION
Receptors have been divided into four major superfamilies: GPCRs, ligand-gated ion channels,
tyrosine kinase receptors, and nuclear receptors. The i rst three receptor superfamilies are located
in the cell membrane and the latter family is located intracellularly.
Our understanding of ligand-receptor interactions and receptor structure has increased dra-
matically during the previous decade, not least due to the rapidly growing number of 3D crystal-
lographic structures that have been determined of either full receptors or isolated ligand-binding
domains. Thus today, structures of partial or full receptors of all four receptor superfamilies have
been determined. Clearly, the information obtained from 3D structures of ligand-binding domains
in the presence of ligands is very valuable for rational drug design (see Chapter 2). Likewise, knowl-
edge about receptor mechanisms can be used to, e.g., design allosteric modulators interfering with
receptor activation.
12.2.1 G P
ROTEIN
-C
OUPLED
R
ECEPTORS
GPCRs are the largest of the four superfamilies with some estimated 1000 human receptor genes.
Approximately 50% of these are taste- and odor-sensing receptors that are not of immediate interest
for the pharmaceutical industry but are of interest, for example, fragrance manufactures. Nevertheless,
it is estimated that 30% of all currently marketed drugs act on GPCRs and the superfamily thus
remains a very important target for drug research. It is fascinating to note the very broad variety of
signaling molecules or stimuli that are able to act via this receptor superfamily, including tastes, odors,
light (photons), ions, monoamines, nucleotides, amino acids, peptides, proteins, and pheromones.
The GPCRs are also referred to as seven transmembrane (7TM) receptors due to the seven
alpha-helical transmembrane segments found in all GPCRs (Figure 12.3) and the fact that the recep-
tors can also signal via G protein independent pathways (see later). The GPCRs have been further
subdivided into family A, B, and C based on their amino acid sequence homology. Thus receptors
Extracellular
Family A
Family B
Family C
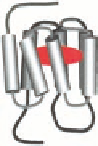
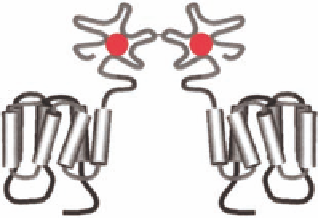
FIGURE 12.3
The superfamily of GPCRs. All GPCRs contain seven α-helical transmembrane segments
and are thus also called seven transmembrane (7TM) receptors. Cartoon of the three families showing the
typical orthosteric binding site (agonist in red); family A receptors bind the agonist in the 7TM region,
family B receptors bind the agonist in both the 7TM region and the extracellular amino-terminal domain, and
dimeric family C receptors bind the agonist exclusively in the extracellular amino-terminal domain. (Adapted
from Ji, T. et al.,
J.
Biol. Chem
., 273, 17299, 1998.)