Biomedical Engineering Reference
In-Depth Information
C-helix
C-helix
AL
C-terminal
tail
AL
C-terminal tail
O
F
Cl
HN
HN
N
O
S
N
N
O
O
O
O
O
O
N
N
(A)
(B)
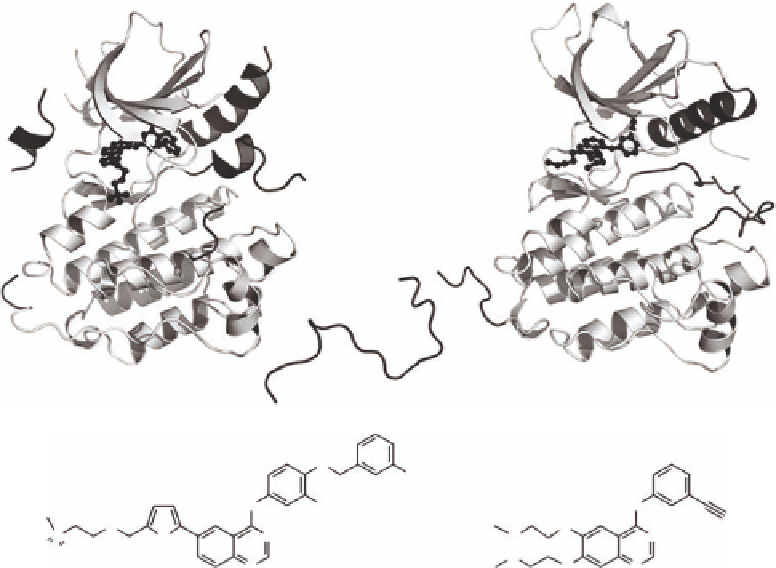
FIGURE 11.8
Protein-ligand binding modes of (A) lapatinib and (B) erlotinib in complex with EGFR (AL,
activation loop). (Courtesy of Lisa M. Shewchuk.)
the 3-l uorobenzyloxy group of lapatinib (Figure 11.8). The structural change is signii cant because
it results in the loss of a highly conserved Glu738-Lys721 salt bridge, which is an important regu-
latory mechanism of kinases, functioning to ligate the phosphate groups of ATP. The net result of
these structural differences is that the activation loop in the lapatinib-EGFR structure adopts a con-
formation that is reminiscent of that found in inactive kinases, while the erlotinib-EGFR structure
displays the activation loop in an active conformation. These effects provide a potential molecular
rationale for the prolonged residence time of lapatinib on its target, which in turn may result in the
observed duration of drug activity in cells. In total, these elegant structural and biochemical studies
have important implications for the discovery of novel, targeted signal transduction inhibitors, and
suggest that subtle differences in kinase inhibitor structure can have a profound impact on the binding
mode, kinetics, and cellular activity.
11. 5.2 S
TRUCTURE
-B
ASED
D
ESIGN
OF
HIV P
ROTEASE
I
NHIBITORS
Perhaps the greatest impact of structure-based design on the identii cation of novel medicines has
been in the treatment of AIDS, the etiologic agents of which are human immunodei ciency virus
type 1 and type 2 (HIV-1 and HIV-2). These retroviruses encode relatively simple genomes consist-
ing of three open reading frames (ORFs),
gag
,
pol
, and
env
. The
gag
gene encodes the structural
capsid, nucleocapsid, and matrix proteins, while the
env
gene is processed by multiple alternative
splicing events to yield regulatory proteins. The
pol
ORF encodes the essential viral enzymes nec-
essary for viral replication: RT, integrase, and protease (PR). HIV-1 PR is an aspartyl protease
that is required for proteolytic processing of the Gag and Gag-Pol polyprotein precursors to yield