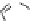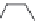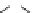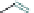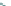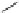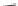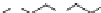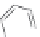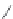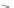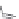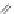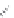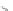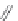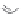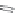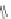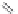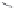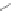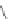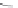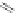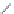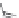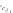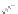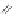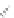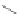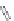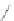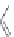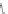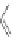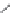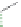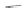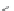Biomedical Engineering Reference
In-Depth Information
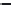
Side chain modiications at His, Phe, and Trp
Peptoid
HN
HN
H
3
C
O
O
O
O
N
N
OH
H
2
N
N
H
2
N
OH
N
N
H
H
O
O
O
O
N
HN
N
N
NH
NH
H
3
C
HN
NH
2
HN
NH
2
HN
HN
O
O
N
H
2
N
OH
Other examples lead to ...
Beta-peptides
Pep
t
ide nucleic acids
H
H
O
N
O
O
OH
O
N
HN
X
H
H
2
N
O
O
H
NH
N
HN
HN
NH
2
NH
O
HN
NH
2
P
H
X = NH, O, or CH
2
(backbone modiication)
HO
O
CH
3
S
C
S
H
Chain elongation
H
N
N
H
H
O
O
S
Amide bond replacements
(backbone modiication)
Single chain replacements
(backbone modiication)
FIGURE 8.5
The most frequent modii cations employed to the peptide backbone.
H
2
N
O
NH
NH
HN
O
O
O
O
HN
O
OH
NH
NH
O
MT II
NH
O
N
H
O
N
N
O
H
2
N
H
N
H
HN
H
2
N
NH
O
O
O
HN
H
2
N
SS
O
O
NH
NH
DPDPE
HO
O
HN
O
O
O
HN
O
NH
NH
O
NH
N
O
H
R
5
R
1
O
R
3
O
N
N
OH
O
H
H
H
2
N
H
2
N
NH
N
H
O
O
HN
NH
O
R
2
R
4
HN
H
2
N
O
O
SHU9119
H
HO
HN
O
NH
O
O
O
H
HN
H
2
N
O
O
O
n
(H
2
C)
O
Enkephalin mimetic
OH
O
c(RGDfV)
FIGURE 8.6
Examples of converting biologically active linear peptides to potent cyclic peptides.