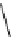Biomedical Engineering Reference
In-Depth Information
and modii ed to obtain insights into the structural and conformational properties that can lead to
peptidomimetics. In this regard, we should begin by carefully dei ning our terms. A “peptide” is
a biopolymer made up of amide (peptide bond) linked
-amino acids. A “peptidomimetic” is a
derivative of a peptide that possesses modii cations of a common peptide structure including pep-
tides containing
α
-amino acids; peptoid structures; amide bond replacements in their struc-
ture; side chain-modii ed structures such as
β
- or
γ
-amino acids; unusual side
chain cyclizations including lactam bridges, lactone bridges, alkane, or alkene bridges, and other
side-chain-to-side-chain bridges; side-chain-to-backbone and backbone-to-backbone cyclizations.
Nonpeptide peptidomimetics whose design is based on peptides will not be discussed here, nor will
so-called peptide mimetics that are discovered as part of some screening processes of chemical
libraries. In any case, these compounds often do not truly mimic the peptide structure related to its
pharmacophore and often when examined carefully have different biological activity proi les.
β
-alkyl or aryl-substituted
α
8.2 GENERAL FEATURES OF PEPTIDE STRUCTURE
Peptides are short biopolymers naturally synthesized from
-amino acids, linked by an amide bond,
also called a peptide bond. The features of a peptide structure or backbone, encompasses critical
information about the backbone torsional angles
α
φ
,
ψ
, and
ω
(Figure 8.1) were i rst investigated
extensively by Ramachandran and coworkers, and
angles were carefully examined later.
The backbone torsional angles dictate the conformational space that is occupied by the peptide
and are the most decisive feature of a bioactive peptide that exhibits afi nity and biological activ-
ity toward receptors such as G protein-coupled receptors (GPCRs), proteases, or other proteins. In
addition, side chain torsional angles (
χ
χ
) play crucial roles in protein-protein folding and peptide
ligand-receptor interactions.
Synthetically designed
-turns, and modii cations of them, are important
considerations for the design of a potent ligand/peptide. Mimicking or replacing atoms or bonds
can introduce conformational constraints on the peptide structure, which can greatly affect the bio-
logical activity. Tuning such modii cations forms the central theme for designing potent ligands for
research and development and for designing peptide-based drugs.
α
-helices,
β
-sheets,
β
ψ
ω
χ
C
o
H
O
C
α
N
N
ω
C
α
C
o
N
C
β
Cα
Cα
N
C
N
ψ
C
o
C
α
N
C
α
O
H
R
H
χ
C
o
N
C
χ
C
α
i
i
-1
i
+1
C
o
represents carbonyl carbon
FIGURE 8.1
Dei nitions of peptide backbone (ϕ, ψ, and ω) and Chi (χ) conformations.
8.3 DESIGN CONSIDERATIONS
The paradigm that underlies biological active peptides and peptidomimetics drug design can
broadly be considered as ligand-based drug design and receptor/acceptor biological activity-based
drug design (Figure 8.2).
For GPCRs, cytokine receptors, and the like, endogenous ligands have afi nity for the extra-
cellular region of the receptor, binding to a specii c region of the receptor. For GPCRs, a ligand
whose binding causes a measurable increase in basal activity of second messengers is dei ned as
an agonist. A ligand that interacts with the receptor in the same binding pocket and causes no