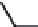Biomedical Engineering Reference
In-Depth Information
N
-Man
OH
NH
2
OH
+
HO
HN
NH
O
H
R
OH
OH
H
O
OH
H
+
N
H
2
N
O
NH
HN
HN
H
O
D-Tyr
NH
HN
O
H
NH
HN
HO
Mephe
(2
S
, 3
S
)
L-Ser
O
Gly
O
MIC ( g/mL)
S. aureus
ED
50
(iv, mg/kg)
Mouse,
S. aureus
Congener
R Group
OH
OH
OH
O
OO
α
>64
20
HO
O
OH
OH
OH
OH
OH
OH
O
OO
HO
O
OH
γ
8
3.5
OH
O
O
OH
OH
OH
O
OO
O
δ
4-8
2.6
HO
O
O
OH
OH
OH
O
OH
O
O
O
ε
4
0.6
O
OH
HO
O
OH
OH
O
708
0.5-1
0.04
OH
O
O
O
O
HO
O
OH
OH
OH
FIGURE 6.14
Structure-activity relationships in the mannopeptimycin antibiotics.
signii cant effect on potency with the epsilon component having the ester at the 4¢-position being
the most potent. These data provide key insights for the design of semisynthetic and biosyntheti-
cally derived analogs in a lead optimization program. In the case of mannopeptimycin this initial
natural SAR led to the semisynthesis of numerous lipophilic derivatives on the terminal di-mannose