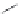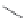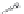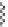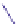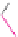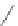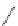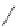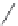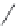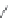Biomedical Engineering Reference
In-Depth Information
H
+
NH
+
L
-Lysine
COOH
H
NAD(P)
+
rapL
L
-Pipecolate
(a)
HO
rapL knockout
OH
Control
N
H
O
OH
OH
OCH
3
OCH
3
HO
O
O
OH
OO
OH
N
N
O
O
O
O
O
O
H
3
CO
H
3
CO
O
O
HO
HO
OCH
3
OCH
3
O
O
(b)
(c)
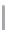
FIGURE 6.11
Mutasynthesis of rapamycin analogs. (a) Conversion of lysine to pipecolic acid mediated by
rapL. (b) Incorporation of pipecolate in control strain leads to rapamycin. (c) Feeding 4-hydroxyproline to the
rapL knockout stain yields a novel analog.
Genes
eryAI
,
ORF C
eryAII
, ORF B
eryAIII
, ORF A
DEBS1
DEBS2
DEBS3
Load
Module 2
Module 4
Module 6
Module 1
Module 3
Module 5
ER
Proteins
DH
KR
ACP
KR
KR
KR
KR
AT
ACP
KS
AT
ACP
KS
AT
ACP
KS
AT
ACP
KS
AT
KS
AT
ACP
KS
AT
ACP
TE
S
S
S
S
S
S
S
O
O
O
O
O
O
O
HO
O
HO
HO
HO
Poly-
ketides
HO
HO
O
HO
O
HO
HO
O
OH
HO
HO
O
O
OH
HO
HO
O
OH
HO
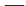
TE = Thioesterase
6-Deoxyerythronolide B
FIGURE 6.12
Polyketide pathway for the biosynthesis of erythromycin.