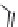Biomedical Engineering Reference
In-Depth Information
HO
HO
OH
OH
O
O
+
D
-Hydantoinase
D
-
N
-carbamoylase
O
HN
HN
NH
NH
H
H
H
2
N
CO
2
H
H
2
N
H
CO
2
H
O
O
Alkaline pH
or
Hydantoin racemase
FIGURE 5.15
Industrial process for the production of (
R
)-
p
-hydroxyphenylglycine.
an appropriate enzyme for a particular chemical synthesis and also to adapt the enzyme to specii c
reaction conditions. Several antibiotics based on the penicillin or cephalosporin structure contain
(
R
)-4-
p
-hydroxyphenylglycine as the acyl side chain. This amino acid is produced on a multithousand
tonne scale by dynamic kinetic resolution of the corresponding hydantoin (Figure 5.15).
Cell-free enzyme preparations offer advantages in simplicity of use, as well as tolerance to harsh
conditions but suffer from the disadvantage of being expensive and may also need additional cofactors
to function correctly. However, whole cells are relatively cheap and all necessary cofactors are present
but product isolation can be complex and side reactions may also occur due to the presence of other
enzymes in the cells. Fermentation using whole cell preparations is used extensively for production of
bulk quantities of drugs such as antibiotics, steroids, ephedrine, cyclosporin, and vitamin B12.
5.6 ANALYTICAL METHODS OF DETERMINING PURITY OF STEREOISOMERS
A number of methodologies are now available for the accurate assessment of enantiomeric purity, which
is for instance, critical for all phases of the drug discovery process. It is standard practice to use more
than one analytical method to determine enantiomeric purity. Two analytical methods have already
been discussed: chiral HPLC (Section 5.5.2) and optical rotation (Section 5.2), and alternative methods
are briel y summarized in the following sections. Readers are referred to the reading list at the end of
the chapter for further details.
5.6.1 N
UCLEAR
M
AGNETIC
R
ESONANCE
S
PECTROSCOPY
Enantiomers cannot be distinguished by nuclear magnetic resonance (NMR) spectroscopy and so
this method relies on the formation of diastereoisomers by the addition of a chiral agent to a mixture
of enantiomers.
5.6.2 G
AS
C
HROMATOGRAPHY
Chiral gas chromatography (GC) has emerged as an extremely efi cient and sensitive method for
the determination of the enantiomeric purity of chiral drugs. In addition, it is now possible to effect
preparative separations of volatile racemic compounds. The main limitation of this technique is
that the sample needs to be readily vaporized without decomposition. However, there are a number
of advantages to the use of chiral GC such as the ability to analyze multicomponent mixtures of
enantiomers and to separate the enantiomers away from trace contaminants. It is possible to extend
the detection of enantiomeric impurities down to the picogram level enabling the reliable determi-
nation of ee to levels >99.9%.
Chiral GC relies on the use of a CSP of high enantiomeric purity to effect resolution of mixtures
of enantiomers.