Biomedical Engineering Reference
In-Depth Information
Figure
2.11.
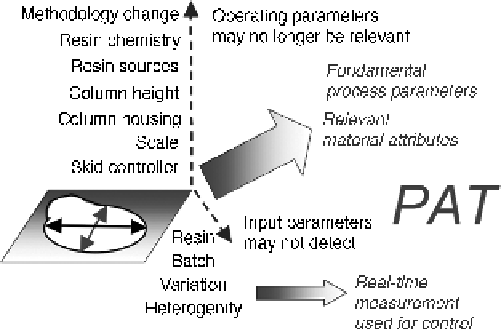
Moving beyond process parameters. The design space described in Fig. 2.10 and
earlier is definedentirely by operatingparameters thatwouldbe specific to the chromatography
methodology and resin used. A wider range of potential changes is shown on the vertical axis
extending from a design space. Dealing with such changes requires parameters that are less
dependent on equipment, such as fundamental process parameters and relevant material
attributes. In addition, even an excellent design space, based on appropriate risk assessment,
experiments, and modeling, may not capture heterogeneity in inputs. Real-time measurements
may capture input heterogeneity. However, real-time measurements of relevant material
attributes are important characteristics of PAT.
The vertical axis lists some of these changes. These can vary from the chromatography
skid and associated pumps and controllers, with easily defined performance criteria, such
as pressure and flows, to a completely different methodology for removing impurities
and inactive charge variants. In between these extremes are changes fromcolumn scale to
resin chemistry. In addition to intentional process changes, there may be unexpected
input changes that would be difficult to detect, such as heterogeneity in a batch of resin.
For large changes in methodology, a design space of operating parameters would no
longer be relevant. However, a design space of fundamental parameters, dimensionless
variables, and ideally, relevant in-process material attributes may allow for such changes.
For unexpected variability in inputs, real-time measurements could facilitate control.
If in-process material attributes and real-time measurement control are added together,
they equal PAT, a system for designing, analyzing, and controlling manufacturing
through timely measurements (i.e., during processing) of critical quality and perfor-
mance attributes of raw and in-process materials and processes [3]. PAT approaches
would allow the maximum manufacturing flexibility.
However, for biotechnology products, process steps often have many functions.
In our hypothetical chromatography example, three outputs were used to characterize the
column performance, basic variants, acidic variants, and an impurity. In reality, a column
could remove additional impurities and play a role in process viral clearance. All the
important process step functions need to be considered in generating a design space. One
means to identify the functions of a process step is to characterize the attributes of a