Biomedical Engineering Reference
In-Depth Information
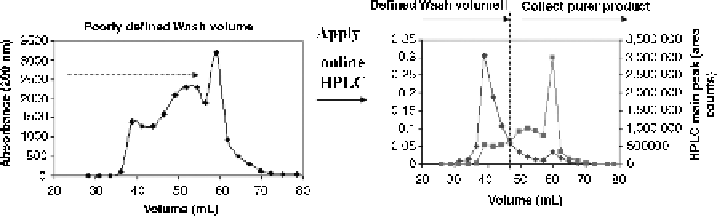
Figure
12.5. Online reverse-phase HPLC for ion exchange fraction pooling to monitor removal
of low molecular weight impurity.
demonstrate that the ability to use the data from the reverse-phase separation to define the
wash volume based on removal of low molecular weight impurity instead of a
predetermined number of column volumes (CVs) used in the wash step (Fig. 12.5).
In a separate set of experiments, Lanan, Kiistala, and Parikh [38] demonstrated the
ability to monitor a protein pegylation reaction to completion by size exclusion
chromatography. A column switching valve was placed between a sampling probe
outlet line from stainless steel pegylation reactor vessel and the analytical HPLC. A
peristaltic pump was used to draw sample from the vessel into the HPLC tubing into the
sample loop on the rheodyne injector valve. The sample loop was switched in-line at
predetermined periodic intervals and a size-based separation was performed from the
beginning of the pegylation reaction to completion. Visualization of the protein of
interest, the monopeglyated species of the protein, and the multipegylated species of the
protein can be achieved by plotting stacking the chromatograms from earlier sampling
points on top of chromatograms from later sampling points (Fig. 12.6a).
By plotting all peak areas from the multi-PEG, mono-PEG, and the raw material
PEG, Lanan, et al. were able to visualize a real-time assessment for the completion of
reaction. With the batch of polyethylene glycol used in this experiment, pegylation of the
protein was complete in approximately 10 h (Fig. 12.6b). This specific application of a
PAT tool would allow the process decision to be made on the basis of an “end point”
analysis, rather than a set time period. If rawmaterial variability in such reactions were a
concern, then having a window into the process might be considered advantageous, as it
would lead to the same product quality regardless of the raw material variability.
In addition, flow injection analysis (FIA) is making a comeback as a PAT tool for
purification. Almeida et al. [45] used FIA to demonstrate the ability to make Fusarium
solani pisi cutinase assessments from an expanded bed absorption eluate using micro-
encapsulation of p-nitrophenylbutyrate (p-NPB) in a micellar system. They were able to
distinguish slight differences in yeast cultivation conditions during cutinase production
that influenced the fermentation performance that affected the adsorption of cutinase
during resin loading. They demonstrated a good correlation between the FIA system
results and the off-line cutinase activity results. Putting this type of system in-line and
using either principles of hydrodynamic chromatography or field flow fractionation may
allow process engineers to assess purification fractions based on larger molecular weight
heterogeneity without the addition of a stationary phase. This approach would also solve