Biomedical Engineering Reference
In-Depth Information
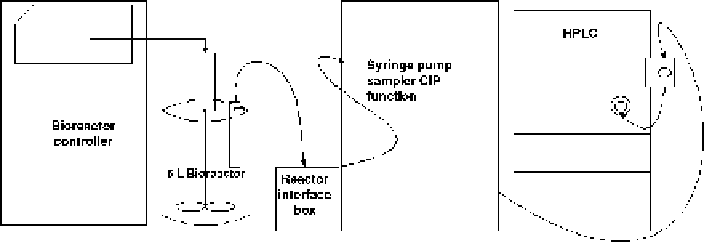
Figure
12.4.
Development lab-scale online HPLC configuration for monitoring critical process
parameters and some critical quality attributes.
for the process developer to understand how the bioreactor conditions, feed media
content, and feed times affect these critical quality attributes. Design of experiments
(DOEs) coupled with multivariate regression tools are typically used to determine the
critical process parameter with the highest contribution to the desired (or undesired)
critical quality attribute. In general, online sampling devices coupled to two-dimensional
HPLC allows the bioengineers to develop a first dimensional separation that focuses on
purification of the protein of interest from the feed stream and a second dimensional
separation that focuses on the product attribute of interest. The same logic applies to
process analytes of interest such as looking at trace elements and sugars by ion
chromatography, although the in-line sample preparation becomes critical. In reality,
the ability to deliver samples from bioreactors straight into empty HPLC vials allows a
variety of derivatization chemistries to be performed by using the injector programming
features present in most modern HPLC autosamplers. These samples may then be
separated and detected byUV, fluorescence, or mass spectrometry. While appropriate for
gaining process understanding in a development lab, the state of these systems, as they
presently exist, is rarely implemented in commercial production bioreactors due to the
complexity of operation, maintenance of the equipment, and impact on the manufactur-
ing process if the instrument fails.
In development labs, the output data from these in-line sterile sampling systems
coupled to traditional analytical devices are currently being input into PLCs through
standard system connectivity languages such as OPC (open connectivity). The PLCs
that receive the information control peristaltic pumps that are actuated when set points are
reached. Based on the incoming data stream from the online analyzer, once a set point is
reached for a given critical process parameter, the PLC activates the peristaltic pump to
begin pumping feed media into the reactor, thus semiautomating a fed-batch process.
At this time, there are relatively few commercial systems available to perform these
types of tasks in a GMP biologics manufacturing environment. At present, only a handful
of equipment manufacturers make HPLCs suitable for the manufacturing floor, and there
are even fewer types of sterile sampling systems necessary to perform automated
sampling. As drug manufactures look for more “relevant time” process analytics related
to process and product, it is expected that more commercial systems will become
available for GMP use in near future.