Biomedical Engineering Reference
In-Depth Information
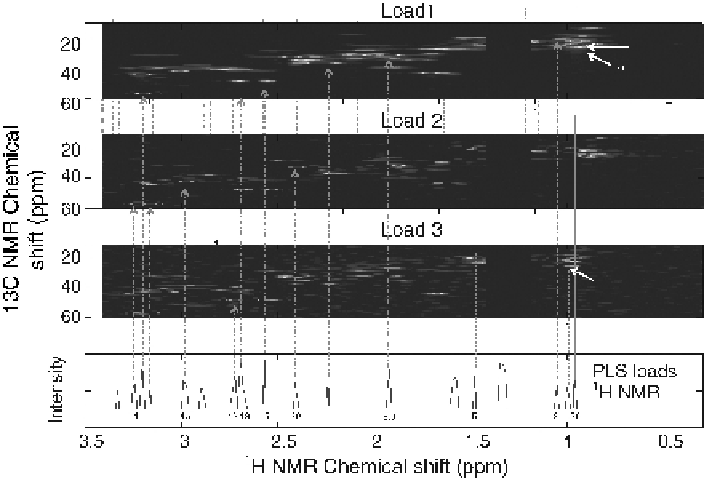
Figure
11.8.
Comparison of three loadings frommultiway PCA of HSQC with
1
H NMR loadings
from a PLS analysis. Peaks that contribute significantly to the PLS analysis of
1
H
NMR can be
identifiedintheHSQCNMR loads. Identifiedcompounds: 2, valine; 4, cadaverine; 5, freealanine; 7,
lysine; 8, N-acetyl glutamic acid; 9, arginine; 13, citrate; 14, choline; 26, leucine; 29, acetyl
phosphate. (See the insert for color representation of this figure.)
are underway to recast these types of data into a Bayes system to understand better the
true risk to future manufacturing.
11.8 CONCLUSIONS AND FUTURE PROSPECTS
The complexity of biological drug manufacturing increases the need to reconsider raw
material acceptance strategies in the light of QbD. An updated testing and release
strategy for raw materials in manufacturing could result in a more robust manufacturing
process. It should include a process to periodically assess and remove analytical tests that
do not add value, maximizing both information and simplicity.
Current practices for raw material analysis demand ID testing on all incoming raw
materials, but too often give no information on suitability of use with respect to
performance. This chapter described a number of upcoming analytical techniques
including HPLC fingerprinting, NMR analysis, ICP-MS, and LC-MS and described
experience managing these methods and results as part of a retrospective investigation.
The challenge, if raw material QbD is to be a truly meaningful phrase, will be in
translating retrospective knowledge into a forward looking, adaptable strategy that can