Biomedical Engineering Reference
In-Depth Information
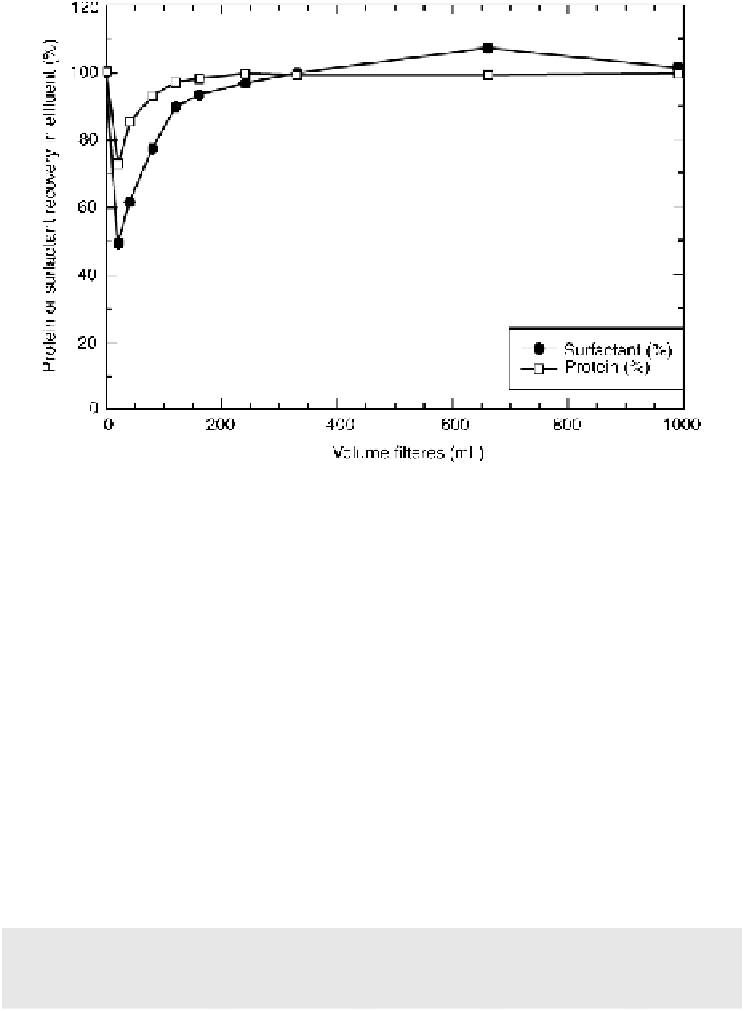
Figure
10.7.
Results of filter sorption study to verify filter selection.
eventuality. In other situations, such a study has been used to define discard volumes after
machine stoppage.
Case Study 3: Compatibility with Infusion Components.
Section 2.6 of
ICH Q8 states “The compatibility of the drug product with reconstitution diluents (e.
g., precipitation, stability) should be addressed to provide appropriate and supportive
information for the labeling. This information should cover the recommended in-use
shelf life, at the recommended storage temperature and at the likely extremes of
concentration. Similarly admixture or dilution of products to administration (e.g.,
product added to large volume infusion containers) might need to be addressed.” This
aspect of the drug product is placed in Focus Area 9 of the process map (Fig. 10.3).
TABL E 10.6. Assessment of Filling Machine Stoppage on Product Composition
Cumulative
Flow of
Bulk (L)
Filter Pre-
Pressure
(bar)
Recovery
Surfactant
(% of Bulk)
Recovery
Protein
(% of Bulk)
Sampling Time
Start of test
2
0.5-0.7
—
—
Before machine stop
(after 0.3 h of process)
4
0.5-0.7
100
100
After machine stop
(after 2.3 h of process)
4
—
95
100