Biomedical Engineering Reference
In-Depth Information
Focus Area 1.
Drug substance
preparation/handling (thawing)
Focus Area 2.
Component preparation
Focus Area 3.
Compounding
In-process testing is
considered part of
Compounding and
Filtration
Focus Area 4.
Filtration
Focus Area 5.
Filling and stoppering
Focus Area 6.
Capping
Focus Area 7.
Inspection
Focus Area 9.
Post production
compatibility
Focus Area 8.
Shipping and storage
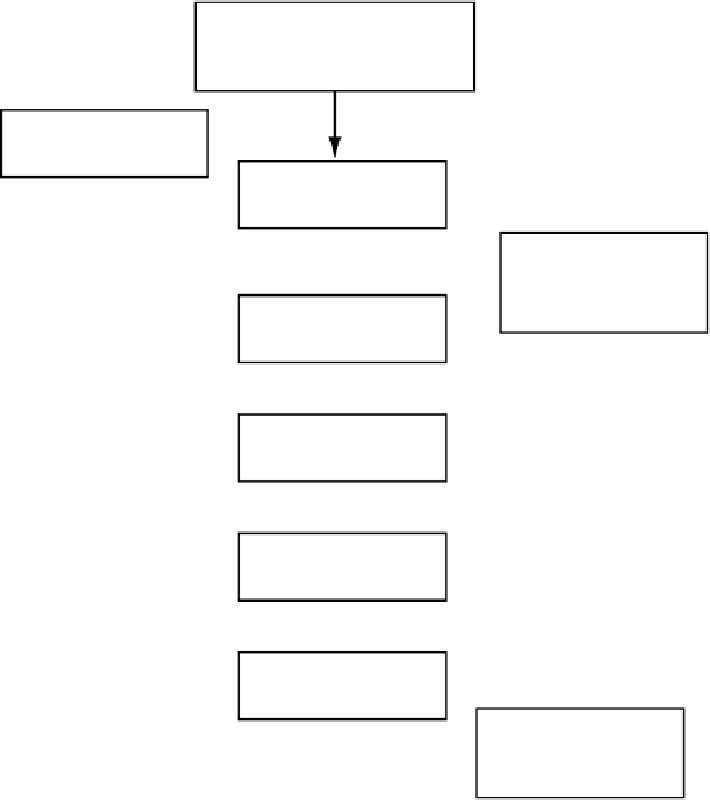
Figure
10.3.
An example of a drug product process map.
(FA3) and filtration (FA4) unit operations. FA5 is filling and stoppering. FA6 is capping
followed by inspection (FA7) and shipping/storage (FA8). Postproduction compatibility,
such as in-use stability, is included in this process as FA9.
After the identification of the focus areas, the entire process map is complete. Each
focus area is then detailed by the specific activities occurring in that unit operation.
Consider compounding, FA3. The compounding unit operation consists of weighing
each component, adding them in order, mixing, and confirming pH and concentration of
the active. Confirmation of pH and concentration is part of in-process testing. Figure 10.4
delineates the compounding step or FA3 in our case study.