Biomedical Engineering Reference
In-Depth Information
TABL E 9.4. (
Continued
)
Factors
Selection Rationale
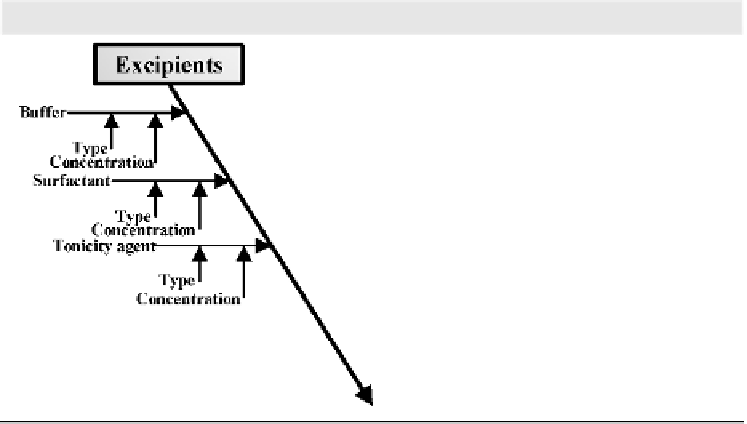
Excipient such as surfactant
stabilizer is selected to effectively
minimize physical instability
issues. The optimal level of
excipients can be determined by
DOE results summarized in the
context of formulation design space
Tonicity agent is selected based on
preformulation and formulation
DOE results with better chemical
and physical stability properties
prototype stability results are compared to the statistical model prediction to update and
finalize the stability risk assessment.
9.10
SUMMARY
The design of a commercial formulation composition in accordance with the Quality by
Design principles generally involves the following steps:
.
Identification of target commercial drug product profile.
.
Preformulation and forced degradation studies to characterize molecular stability
properties, impact of formulation variables, and other factors.
.
Preliminary stability risk assessment with emphasis on direct impact on the
activity based on preformulation and forced degradation studies results.
.
Initial formulation risk assessment to establish the cause-effect relationship of
different factors and solution formulation stability via the Ishikawa (Fishbone)
diagram.
.
Multivariate DOE studies to optimize the formulation composition and define a
robust design space to meet the expected shelf life of 24 months at 5
C.
.
Establish formulation design space based on DOE results and stability properties
projections.
Select commercial solution formulation based on design space, molecule knowl-
edge, and risk assessment.
.