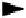Biomedical Engineering Reference
In-Depth Information
Acidic pH
Neutral pH
Alkaline pH
Chemical stability (e.g., CEX-HPLC)
Chemical stability (e.g., SEC-HPLC)
Physical stability (e.g., turbidity A550)
+ Surfactant
Physical stability (e.g., turbidity A550)
Thermal stability (e.g., DSC)
Solubility
Optimal pH
Figure
9.3. Example of balancing analytical properties to identify optimal pH.
9.7 INITIAL FORMULATION RISK ASSESSMENTS
As stated earlier, a significant hurdle to successfully develop a stable solution formula-
tion is mostly derived from the multiple degradation pathways and chemical modifica-
tions located in different regions of the molecule. A preliminary stability risk assessment
should take into consideration the impact of these modifications on the critical quality
attributes directly linked to the TPP, as well as the stability growth potential.
In the case of a monoclonal antibody, modifications located in the variable region
(Fig. 9.2) known to have impact on the activity are considered to have high stability risk
and need to be characterized and carefully monitored during stability studies. For those
chemically modified forms without stability growth potential, the risk factor should be
initially assigned as mediumuntil the stability trend is fully characterized later. Chemical
modifications in the constant region with no impact on the binding activity and no
stability growth potential are considered to have low risk. However, a medium risk factor
is assigned when there is stability growth potential where the rate constants need to be
monitored to finalize the risk factors. Another important consideration is aggregation,
which is considered to be of high risk due to the potential impact on the drug product
performance with respect to the binding activity and immunogenicity effect. In addition,
aggregation usually grows on stability and hence needs to be carefully characterized and
monitored during stability studies. The type and location of each modification, as well as
the potential impact on critical quality attributes and associated stability risk, are
summarized in Table 9.3.
A second element of the initial formulation risk assessment investigates possible
impact of formulation parameters and conditions, such as pH, excipients, trace metals,
light, and so on, on solution formulation stability. Based on preformulation and
characterization experience, as well as common protein chemistry and parenteral
knowledge, the factors assessed are shown in the cause-effect Ishikawa (Fishbone)