Biomedical Engineering Reference
In-Depth Information
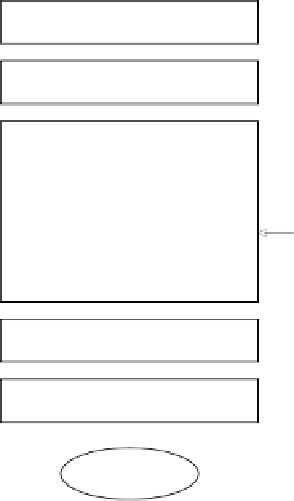
Seed vial thaw
Inoculum development
15 or 300 L fermentation
Batch growth
Feed 1
Fed-batch growth
Induction (production)
Feed 2
Chill for harvest
MF/DF + Centrifugation
Cell paste
Figure
5.2. General process flow diagram for the case study.
an interdisciplinary team consisting of representatives from process/analytical devel-
opment and manufacturing. The parameters are assessed with respect to the severity of
operational parameter excursion (S), frequency of occurrence of the excursion (O), and
ease/difficulty of detection of the excursion (D) [5]. Then, a risk priority number
(RPN
D) score is calculated and used in the prioritization process.
In the case study shown in Fig. 5.3 and based on the review of the RPN scores,
parameters exhibiting an RPN score
¼
S
O
20 were considered a risk high enough to merit
further characterization. These included the inoculum density for the seed fermenter
step; acid/base control and dissolved oxygen (DO) for the growth phase of production
fermenter; and optical density for induction and dissolved oxygen for the production
phase of production fermenter. Some parameters with lower RPN scores, for example,
pH and temperature during growth and induction phase, were included for characteri-
zation due to their high severity scores so as to generate process understanding for future
process improvement efforts.
>
5.4 SMALL-SCALE MODEL DEVELOPMENT AND QUALIFICATION
A qualified small scale model is critical to process characterization, process-fit studies,
manufacturing troubleshooting, and process improvement studies [4, 5, 7]. An accept-
able small scale model needs to not only represent the large-scale performance at
operating set point but also in the operating range and beyond (Fig. 5.4). This ensures that
the results generated from the process characterization studies are applicable to the
process at manufacturing scale. Figure 5.5 illustrates an approach toward small-scale