Biomedical Engineering Reference
In-Depth Information
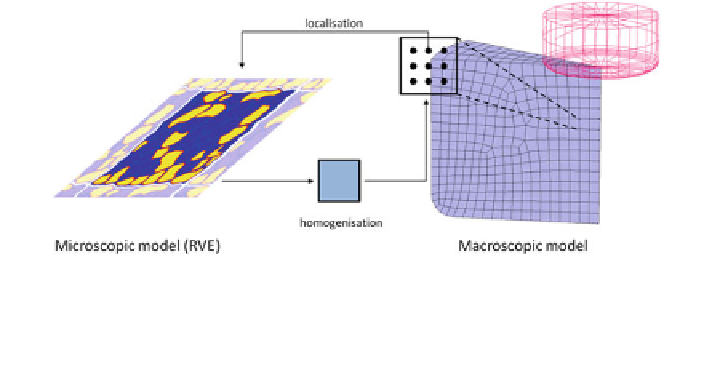
Fig. 6 Schematic illustration of the multiscale approach. Instead of a constitutive model at the
integration point level of the macroscopic domain, a localisation rule is used to determine the
boundary conditions for a representative volume element (RVE, here example with periodic
boundary conditions [
82
]). The solution of the microscale problem is then homogenised
(smeared) and passed up to the macroscopic level again. Colour version available online
indispensable tool in this process [
104
]. Another challenge is to bridge the scales
and integrate the information into multiscale models that are predictive across a
range of scales. This presents challenges on all levels: experimental, theoretical
and computational. The computational methods for scale bridging and coarse
graining have not come to a close and neither have mechanoregulation theories for
tissue differentiation. Thus, while a significant body of literature exists at both
ends, little has been done at the interface, i.e. multiscale mechanobiological tissue
differentiation models.
Bone properties are determined by the intrinsic material properties and
morphology of the underlying trabecular structure. Since a single computational
model of a full bone that is detailed enough to capture the underlying trabecular
architecture does not seem feasible, the problem lends itself to hierarchical mul-
tiscale methods. The classic approach (Fig.
6
) is computationally very expensive:
Starting from a deformation estimate on the macro-model, local boundary con-
ditions are derived for the micro-models (following a localisation rule). Finite
element simulations of often complex representative volume elements have to be
then solved for each integration point location of the macroscopic domain and the
stress response is then homogenised and passed up to the macroscopic model
replacing pre-defined constitutive models at this level. This loop has to be iterated
until equilibrium is achieved and only then can the next increment be processed.
For models of a practically relevant size this approach is only feasible on large
parallel computing facilities where the RVE simulations are distributed and solved
simultaneously. Hambli et al. [
42
] introduced neural network computation into
multiscale simulations of bone remodelling. Five lCT based models of the
trabecular structure served as RVEs and a remodelling algorithm [
41
] was
implemented. These RVEs were loaded with boundary conditions derived from the
macroscopic model under known inputs (the amplitude, orientation and frequency
of the applied stress) to derive five outputs (the averaged bone density, damage,
Search WWH ::

Custom Search