Biomedical Engineering Reference
In-Depth Information
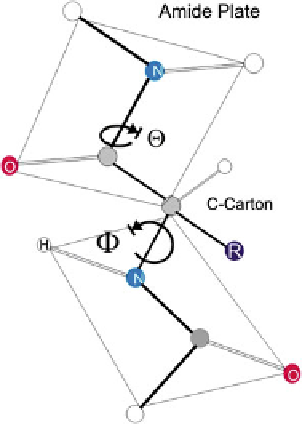
Fig. 1 Rotations around
peptide groups. Two planar
peptide groups are shown in
this illustration. The only
reasonably free movements
are rotations around the
C
a
-ON bond (measured as U)
and the C
a
-OC bond
(measured as H).
By convention, U is 180
and H is 90 in the
conformation shown and
increase, as indicated, in the
clockwise direction when
viewed from C
a
thought of as a backbone with additional groups attached to it. The C-N backbone
is not straight because the bonds are not collinear. For example, carbon forms single
bonds that are spaced equally apart from each other form a tetrahedron angle
(109.5)[
83
] rather than straight chains in the case of the residues.
With the distances between the C-N backbone being non-collinear, the angles
between directional vectors are not parallel. From this, the groups are replaced
with an outline of the atoms centered on the backbone so that we have strings of
beads (though the bead shape is not round [
21
]). The lacing of the beads is the
backbone of the protein is shown in Fig.
2
a. Each of the amino acids has bonds
that can rotate. In most cases there are two bonds that rotate. The R groups (amino
acid side chains) can take one of several states. In the case of proline there is only
one free rotating bond (to the H) [
28
], this is handled by an error function that adds
a large penalty to the optimization function such that the bond will stay at the
optimal angle (Fig.
2
b). Generally, all bonds are considered to be of fixed length
and only rotation is allowed. The angles are the parameter. The volume and
surfaces are the results. The simplex method requires only functions given by the
objective function [
41
,
55
]. The bond lengths never change, the only change
occurs in the two angles per residue in our configuration search [
85
].
The rotation of non-collinear bonds allows the molecule to twist, similar to a
Rubik's cube puzzle toy where a set of angles are joined by rotating joints. This
rotation allows the protein to take a shape [
56
]. The molecule can be twisted to
nearly any shape, but the proper shape is achieved by optimization of an objective
function. The objective function mirrors an energy function.
The method used for the geometrical model is trilateration, which is a type of
measurement that determines a point by using the geometry of spheres, circles, or
triangles. Unlike triangulation, which uses the measurement of angles to determine
Search WWH ::

Custom Search