Biomedical Engineering Reference
In-Depth Information
4.4.4 Improvement in Outcome Measures
Upper limb impairment was assessed before and after the therapy using the
Chedoke-McMaster Impairment Inventory (CMMII) (Gowland
et al.
(1993)), where
the impairment is scaled from stage 1 (severe impairment) to stage 7 (mild
impairment). Robot-assisted training resulted in direct benefits in ADL for the
four subjects, and in a reduction of their impairments. A mean improvement of
+
was observed in the four patients who participated to the
pilot study.
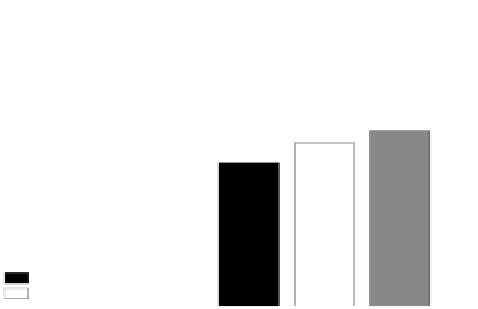
Figure 4.8(A)
presents the evolution of CMMII scores for the four
stroke patients.
In addition, patients reported improvement in their daily activities at home;
they felt more secure in grasping and manipulating objects, more skillful in fine
motor tasks such as manipulating buttons or handwriting, and started to use their
impaired hand more, which we believe is crucial for further improvement.
These preliminary results motivated a larger clinical study with the Haptic-
Knob. Nine subjects
1.05 stages
(+
25%
)
12.3 years, 5 females) at the chronic post-stroke stage,
3 right and 6 left hemiparetic, participated in this study. Subjects participated in a
one-hour session 3 times a week over a period of 6 weeks, resulting altogether in
18 sessions during which they trained with the HapticKnob. Evolution in arm and
hand motor function was assessed using clinical tests including the Fugl-Meyer
arm motor scale (FM, range [0-66], (Fugl-Meyer
et al.
(1975))). After 6 weeks of
therapy with the HapticKnob, a mean increase of 4.3 points
(
59.4
±
(+
)
was observed
in the FM scores, with a maximum improvement of 11 points in one of the patients
(
Fig. 4.8(B)
)
, which confirmed positive results obtained in the first study with the
HandCARE and the HapticKnob (Lambercy
et al.
(2009)).
These results suggest that an intensive and repetitive training program im-
proves motor function in chronic stroke subjects long after completion of con-
14%
A
CMMII stage [0−7]
B
FM score [0−66]
7
*
*
p < 0.05
p < 0.001
*
60
**
6
**
50
5
40
4
30
3
20
2
10
1
week 0
week 6
week 12
week 0
week 8
0
0
Figure 4.8
Evolution in clinical outcomes after robot-assisted rehabilitation of hand func-
tion, during the first clinical study with the HapticKnob and the HandCARE (A) and during
the second study with the HapticKnob (B).