Biomedical Engineering Reference
In-Depth Information
Electrolyte:
Li
+
, EC, DEC, PF
6
Li
+
(a)
e
−
External circuit:
e
−
Li
+
+ ntTiO
2
+ e
−
(b)
p
δ
Li
x
ntTiO
2
δ: 50
−
150 nm
p: 20 nm
L: 600
−
900 nm
L
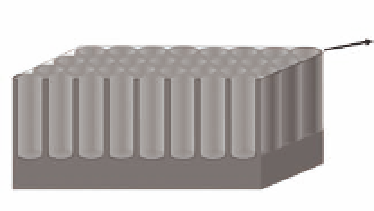
Figure 5.16
(a) Schematic representation of the self-organized titania
nanotubes used for the assessment of the specific area. (b) Transport path
of lithium ions and electrons in mesoporous titania nanotube.
It can also be noted that the ratio between the irreversible
capacity of the amorphous and the annealed materials is around 5.
This difference can be explained by the fact that annealing treatment
of the amorphous electrode removes structural and chemical defects
that act as Li
ion traps, which are responsible for the irreversible
insertion of Li
+
. This explanation is for titania nanotubes with 900
nm, but it can be also applied for the shorter nanotubes show above.
The cycle performance of as-formed and annealed ntTiO
+
-based
2
) is illustrated
in Fig. 5.17 for 50 cycles. First, these results suggest that ntTiO
2
electrodes at different kinetics (5, 20 and 100
μ
A cm
-
2
can be used as an alternative electrode for rechargeable Li-ion
microbatteries. The specific capacity obtained for the as-prepared
samples is higher than for the annealed materials confirming that
the amount of lithium ions inserted into amorphous ntTiO
2
is higher
than in the crystalline structure. The highest specific capacity
is obtained with as-prepared ntTiO
layers using a relative slow
2
or C/8), which delivered a maximum reversible
capacity of 77 μAh cm
kinetic (5 μA cm
-
2
after
50 cycles (see Fig. 5.17a). The average capacity loss of 0.45 μAh cm
-
2
after the first cycle and 55 μAh cm
-
2
-
2




































Search WWH ::

Custom Search