Biomedical Engineering Reference
In-Depth Information
3.0
3.0
(a)
(b)
Amorphous ntTiO
2
(600 nm)
onto Si substrate
Crystallized ntTiO
2
(600nm)
onto Si substrate
50
th
1
st
2.7
2.7
50
th
3
rd
2
nd
1
st
2.4
2.4
2.1
2.1
1.8
1.8
1.5
1.5
1.2
1.2
50
th
3
rd
2
nd
0.9
1
st
3
rd
1
st
50
th
2
nd
0.9
0
20
40
60
80
100
120
140
160
180
200
0
20
40
60
80
100
120
140
160
180
200
220
Areal Capacity /
µ
Ah cm
-2
Areal Capacity /
µ
Ah cm
-2
200
(c)
Si substrate
180
Amorphous ntTiO
2
, 5
µ
Acm
-2
Amorphous ntTiO
2
, 100
µ
Acm
-2
Crystallized ntTiO
2
, 5
µ
Acm
-2
Crystallized ntTiO
2
, 100
µ
Acm
-2
160
140
120
100
80
60
40
20
0
0
10
20
30
40
50
Cycle Number / n
Figure 5.13
Galvanostatic discharge/charge curves during the 50 cycles of
ntTiO
nanotube layers obtained onto Si substrates: (a) as-prepared, and
after thermal treatment at 450°C (b). (c) Evolution of its areal capacity as a
function of cycle number using a kinetic of 5 (C/8) and 100 (2.5C) µA cm
2
−2
.
Other possible reasons for the high experimental capacity
values are the high surface area and highly organized 1D structure
of titania nanotube layers. When a rate of 100
μ
(2.5C) is used,
the reversible and irreversible capacities are lower than at a rate of
5
A cm
−2
μ
, but the capacity retention for crystalline is around 96%
(close and star-shaped symbols in Fig. 5.13c; Table 5.1).
A similar behavior was found in the galvanostatic curves of
ntTiO
A cm
−2
samples with 600 nm length manufactured on commercial
Ti foil (Fig. 5.14). The main difference between amorphous and
crystalline nanotubes resides in the observation of a voltage plateau
during the discharge (1.75 V) and charge (1.95 V) of the cell during
the 50 cycles. Under low kinetics, the amount of lithium inserted
into amorphous (53
2
μ
Ah cm
−2
) is higher than in crystalline ntTiO
2
μ
(49
Ah cm
−2
), and they exhibit after 50 cycles an efficiency of 70%


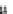

















































































































































































Search WWH ::

Custom Search