Biomedical Engineering Reference
In-Depth Information
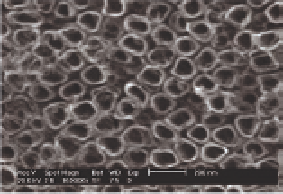
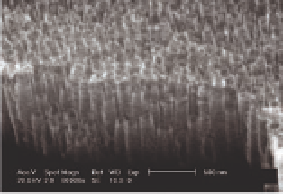
Figure 5.10
Top-view (left) and cross-section (right) SEM images of ntTiO
2
after annealing at 450°C in air for 3 h.
5.4.2 TiO
2
Polymorph: The Interest of Anatase and
Amorphous Titania
polymorphs have been reported and named as rutile,
anatase, brookite, TiO
Several TiO
2
-B (bronze), TiO
2
-R (ramsdellite), TiO
2
-H
2
(hollandite), TiO
2
-II (columbite), and TiO
-III (baddeleyite). Since a
2
long time, the practical use of TiO
2
had been as white pigment in
paint substituting the toxic lead oxides. One of the reasons for this
is its whiteness attributed to the relatively high refractive index,
which, in combination with small particle sizes, results in strong
light scattering in a broad range of wavelengths. Other commercial
applications are in products such as sun lotion and toothpaste due
to its remarkable optical properties and chemical stability and as a
pigment when the crystalline form has a high refractive index (rutile).
Concerning energy conversion and storage applications, titanium
dioxide (TiO
) is one of the most important wide-gap semiconductors
and is widely envisaged for use in photoelectrochemical solar cells,
supercapacitors, and Li-ion batteries [41, 42], among others.
Photoelectrochemical solar cells
2
in solar
energy conversion is directly related with the early development of
photoelectrocemical cells to either water cleavage and H
: The use of TiO
2
production
or electricity generation. In the early 1970s, Fujishima and Honda
proposed a design of solar cell benefiting from the semiconductor
properties of TiO
2
. Its bandgap energy is suitable for adsorbing
ultraviolet-light-promoting electrons to conduction bands. The
potential difference created in the electrode is appropriated to
oxidize oxide anions while H
2
is produced in the Pt counterelectrode
2








Search WWH ::

Custom Search