Biomedical Engineering Reference
In-Depth Information
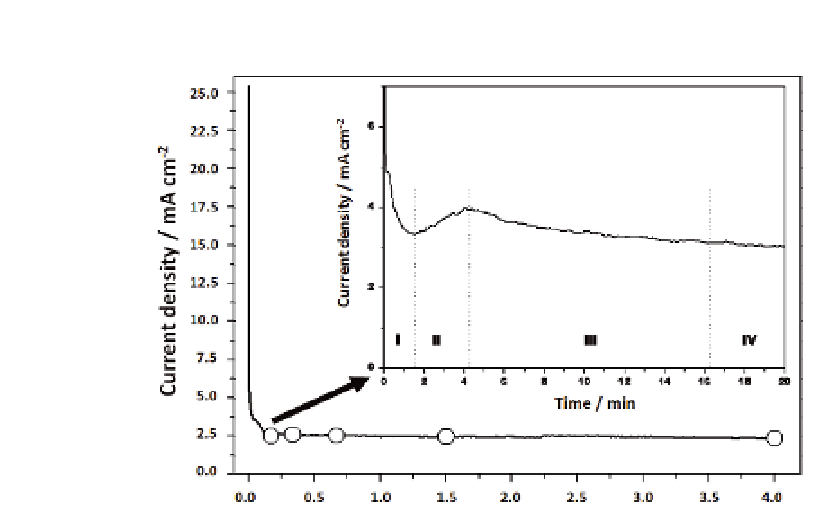
Current-time curves for the 4 h and 20 min (inset) of anodization
under a potentiostatic regime at 20 V in 1 M H
Figure 5.4
+ 1 M NaOH + 0.5 wt%
HF solution. The four distinct phases of anodization are marked with roman
numerals in the inset.
PO
4
3
In fact, the growth and dissolution of the oxide layer continues
but is much slower at this stage. During this stage, the initial compact
layer (that has become porous meanwhile) is completely etched off
the surface of the TiO
2
nanotubes. SEM pictures of the resulting
titanium dioxide nanotubes are shown in Section 5.4.
5.3.2 The Principle of Fabricaion
nanotube
layers [26, 38] have shown that the growth of oxide nanotubes
results from a competition between two reactions. The first reaction
is the electrochemical formation of titanium dioxide and may be
written in two forms:
Several studies on the formation of self-organized TiO
2
Ti + 4H
O - 4e
-
� Ti(OH)
+ 4H
+
(5.1a)
2
4
Ti + 2H
O - 4e
-
� TiO
2
+ 4H
+
(5.1b)
2







Search WWH ::

Custom Search