Biomedical Engineering Reference
In-Depth Information
Besides, polyelectrolyte-functionalized IL (PFIL) also showed
great potential in stabilization of Au NPs [68]. It was found that
the size distribution of resulting Au NPs (PFIL-AuNPs) was narrow
and could be tuned by the concentration of HAuCl
4
. Moreover, they
showed a high stability in water at room temperature for at least
one month in solutions of pH 7-13 (even in the present of high
concentration of NaCl). In addition, the PFIL-AuNPs exhibited
obvious electrocatalytical activity toward NADH oxidation,
suggesting a potential application for bioelectroanalysis (Fig. 4.21).
By further cooperating with electric conductive CNTs, the resulting
nanocomposites (CNT-AuNPs-PFIL) showed obvious electrocatalysis
toward reduction of H
. In addition, when glucose oxidase
was immobilized into CNT-AuNPs-PFIL thin films, it demonstrated
favorable linear catalytic response to glucose [69].
O
and O
2
2
2
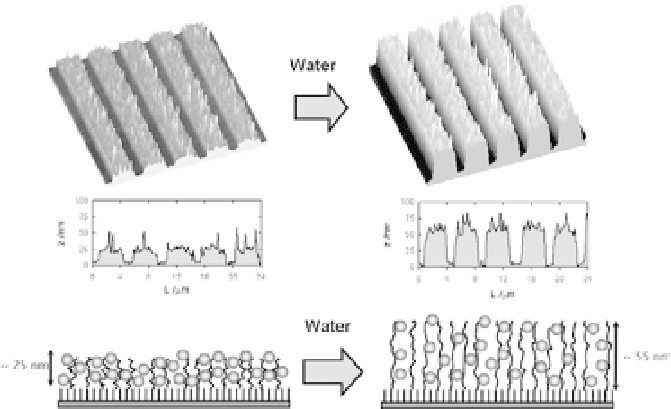
Patterned IL-based polyelectrolytes brushes loaded with
AuNPs, in compressed and swollen states (left and right, respectively).
Top, middle: AFM images and cross-sectional analyses of patterned brush
layers, obtained in air and water; bottom: schematic corresponding to AFM
data [70]. Reproduced by permission of The Royal Society of Chemistry.
Figure 4.22
Huck et al. prepared Au NPs inside IL-based polyelectrolytes [70].
The nanocomposite synthesis relies on loading the macromolecular
film with AuCl
precursor ions followed by their in situ reduction
to Au nanoparticles. It was observed that the nanoparticles
are uniform in size and are fully stabilized by the surrounding
4-








Search WWH ::

Custom Search