Biomedical Engineering Reference
In-Depth Information
4.5 ILs-BASED MULTIFUNCTIONAL COMPOUNDS FOR
ELECTROCATALYSIS AND BIOSENSORS
(a)
(b)
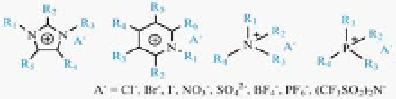
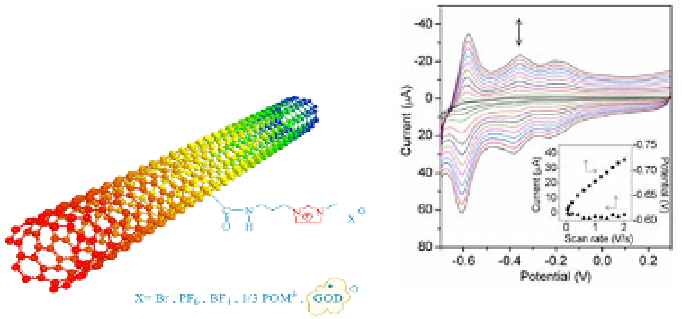
Figure 4.14
(a) Examples of cations and anions commonly used for the
formation of ionic liquids and SWNT-IL-X. (b) Cyclic voltammograms (CVs)
of SWNT-IL-POM modified GC electrode (
d
= 3 mm) at different scan rate
in 0.5 M H
. Scan rate: 0.05, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4, 1.6, 1.8
and 2.0 V/s from inner to outer. The inset shows the peak current (square)
and peak potential (triangle) of the third reduction wave as a function of
scan rate. From Zhang, Y. J., Shen, Y. F., Yuan, J. H., Han, D. X., Wang, Z. J.,
Zhang, Q. X., and Niu, L. (2006). Design and synthesis of multifunctional
materials based on an ionic-liquid backbone.
SO
2
4
, pp.
5867-5870. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced
with permission.
Angew. Chem. Int. Edit.,
45
One of the most fantastic features of ILs is that their properties can
be easily and well tuned by rationally selecting proper combination
of organic cations and anions. That also means that we can delicately
utilize one ionic component to deliver a unique function and the
other ionic component to deliver a different, completely independent
function. Moreover, the components and their combination of
anions and cations are various, for example, the cation of ILs can
have several substituting groups (R1, R2, R3, etc., as shown in
Fig. 4.14a) and these substituting groups are tunable. Therefore, it
offers us a promising and facile way to combine individual functions
into a target compound. In contrast, for a commonly seen compound,
to achieve this multifunctional combination would encounter all








Search WWH ::

Custom Search