Biomedical Engineering Reference
In-Depth Information
The partition coefficient and diffusion coefficient of HQ in the PFIL
film were calculated to be 0.346 and 4.74
×
, respectively.
Electrochemistry in PFIL is similar to electrochemistry in a solution
of traditional supporting electrolytes in the solution, except that the
electrochemical reaction takes place in a thin film on the surface of
the electrode. PFIL is easy to be immobilized on solid substrates,
inexpensive and electrochemically stable. A PFIL-modified electrode
assembly is successfully used in flow analysis at HQ by amperometric
detection in solution without supporting electrolyte. Similarly,
polypyrrole could be electropolymerized on PFIL-PSS modified
electrodes without added support electrolytes [54].
10
-
6
cm
2
s
-
1
(a)
(b)
(c)
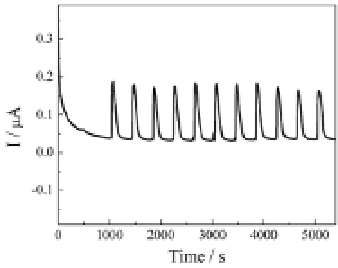
Figure 4.12
Illustration of the electrochemical process of the analytes
at the PFIL-modified electrode assembly in a supporting electrolyte-free
solution (a); amperometric responses of repeated injection of a 20
M HQ
solution (b); amperometric response of HQ at different concentrations.
The signal was recorded at the 1.4 mm-thick PFIL-modified electrode
assembly at +0.3 V. Double-distilled water was used as the carrier solution.
Flow rate: 1 mL min
m
-
(c) [53]. Reproduced by permission of The Royal
1
Society of Chemistry.










Search WWH ::

Custom Search