Biomedical Engineering Reference
In-Depth Information
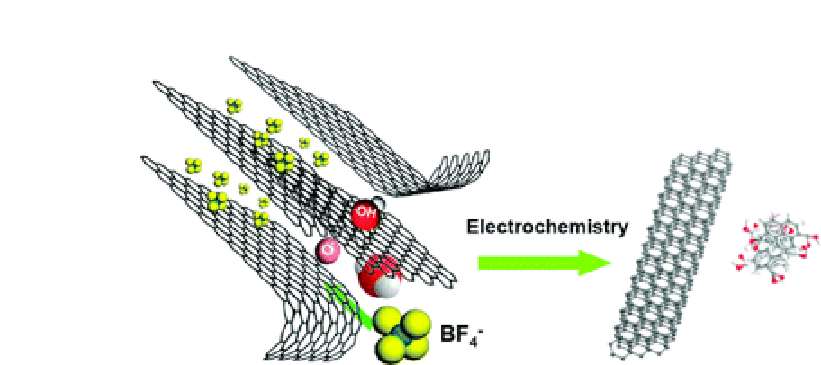
Figure 3.8
Illustration of the exfoliation process showing the attack of the
graphite edge planes by hydroxyl and oxygen radicals, which facilitate the
intercalation of the BF
anion. Oxidative cleavage of the expanded graphite
produces graphene nanoribbons. Reprinted from
−
4
, Lu, J.,
Yang, J. X., Wang, J., Lim, A., Wang, S., and Loh, K. P., One-pot synthesis of
fluorescent carbon nanoribbons, nanoparticles, and graphene by the
exfoliation of graphite in ionic liquids, 2367-2375, Copyright (2009), with
permission from Elsevier.
ACS Nano
,
3
3.5 CONCLUSIONS
Use of ILs in the electrochemical synthesis of organic electroactive
materials opens up new possibilities to improve both quality and
quantity of the produced material. Production of nanomaterials can
even be made in one step since ILs in many cases act as both solvent
and activator in the synthesis. Ionic conductivity of ILs is sufficient
for electrochemistry and the anions are incorporated into the
polymer film during polymerization to preserve electroneutrality.
Both the yield and the rate of polymerization for some conducting
polymers can greatly be improved when performed in ILs. All
studied electrochemical synthesis of conducting polymers except
of PANI resulted in a higher material deposition in ILs than in
conventional electrolytes. The quality of polymer films made in ILs is
also enhanced, showing higher electrical conductivity of the polymer.
ILs provide extended electrochemical windows. Hence, it is possible
to dope the polymers at potential levels unavailable in conventional
solvents. Also films made in ILs (e.g., PPP) exhibit enhanced







Search WWH ::

Custom Search