Biomedical Engineering Reference
In-Depth Information
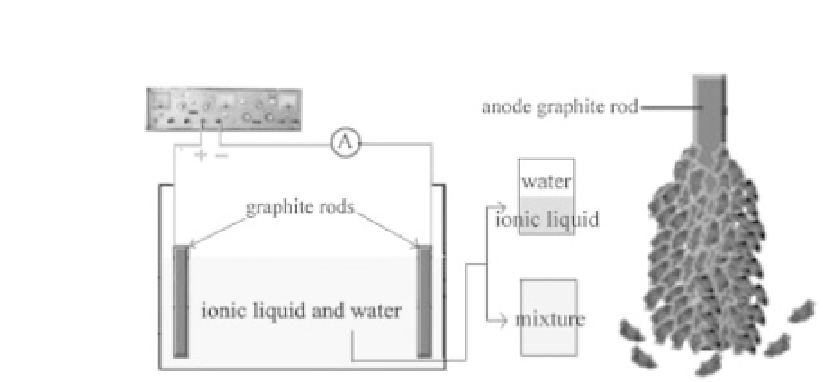
Experimental setup diagram (left) and exfoliation of the
graphite anode (right). Liu, N., Luo, F., Wu, H., Liu, Y., Zhang, C., and
Chen,
Figure 3.7
J.
One-step
ionic-liquid-assisted
electrochemical
synthesis
of
ionic-liquid-functionalized
graphene
sheets
directly
from
graphite.
Adv. Funct. Mater
. 1518-1525. Copyright Wiley-VCH Verlag GmbH
& Co. KGaA. Reproduced with permission.
. 2008.
18
Lu et al. made carbon nanoparticles and graphene from graphite
electrode in ILs [95]. The scope of their research was to make
fluorescent nanomaterials that could be applied in bioimaging
[96]. In their work, [BMIM][BF
] was mixed with water and used
to produce carbon nanoparticles (graphene sheets) in similar
manner as in the studies conducted by Liu et al. [91]. The schematic
presentation of their exfoliation process is shown in Fig. 3.8. They
proposed a strong influence of water on the exfoliation mechanism
since the increase in the water content in IL was found to result in a
decrease in the activation energy required for graphite separation.
As already stated, addition of water (also present as impurity)
decreases electrolyte resistance and results in a decrease in the
potential window. The hydroxyl and oxygen radicals formed during
application of relatively high voltages (ranging, for instance, from
1.5 to 8 V; depending on the water content) facilitate both expansion
(intercalation of BF
4
anion) and corrosion of the graphite electrode.
The resulting precipitate is composed of graphene sheets. It was
shown that by changing the water/IL ratio several, nanomaterials
can be made in different shapes and sizes. By controlling this ratio, it
was possible to generate fluorescent nanomaterials to be used in the
ultraviolet and visible range.
-
4







Search WWH ::

Custom Search