Biomedical Engineering Reference
In-Depth Information
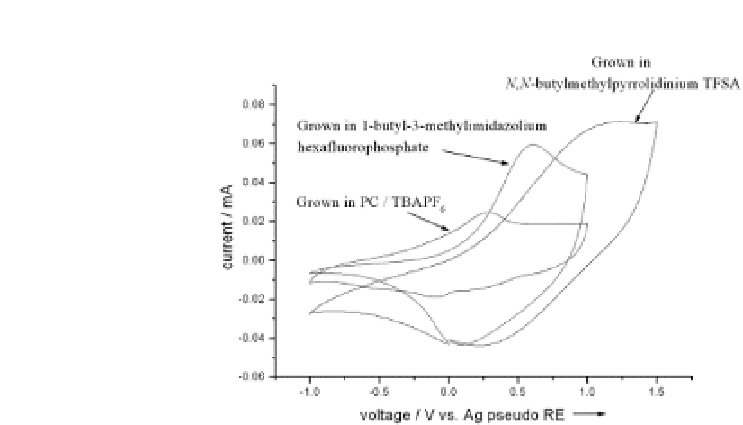
Figure 3.2
Comparison of the electrochemical activity of the polypyrrole
films in PC/TBAPF
solution. Scan rate 100 mV s
. Reprinted from
−1
6
Polymer
, Pringle, J. M., Efthimiadis, J., Howlett, P. C., Efthimiadis, J.,
MacFarlane, D. R., Chaplin, A. B., Hall, S. B., Officer, D. L., Wallace, G. G.,
and Forsyth, M., Electrochemical synthesis of polypyrrole in ionic liquids,
1447-1453, Copyright (2004), with permission from Elsevier.
,
45
Synthesis and doping studies of PEDOT have been conducted
by Damlin et al. with [BMIM][PF
] as the solvents
[55]. They noticed continuous polymer growth and the process
had similar features as reported for electrosynthesis in organic
solvents. The polymerization process was also studied by
] and [BMIM][BF
6
4
in situ
attenuated total reflectance Fourier transform infrared (ATR-FTIR)
spectroscopy. Figure 3.3 presents the changes in the IR spectra with
oxidation potential, showing increase in the band intensities with
increasing potential. The IR peaks observed are similar to the peaks
of PEDOT obtained in organic solvents. However, during p-doping
of the polymer at higher potentials, the intensity of the IR induced
bands was increased compared with the peaks observed at the same
doping potentials in conventional organic solvents. Wagner et al.
studied electropolymerization of PEDOT in [BMP][Tf
N], [EMIM]
2
N] and ACN [56]. They found that the cation of the IL used has
an effect on the polymerization yield since the rate of the synthesis
of PEDOT was faster with EMIM
[Tf
2
as the cation. The authors also
+
made the conclusion that the Tf
anion has an effect on the film
morphology in terms of ion incorporation. Randriamahazaka
N
-
2







Search WWH ::

Custom Search