Biology Reference
In-Depth Information
A
B
C
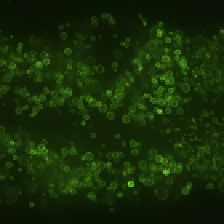
Emission: 500-555 nm
Emission: 580-650 nm
Merge
FIGURE 3.3
Visualization of GFP::DGAT-2 by spinning disk confocal microscopy. A young adult animal
was imaged. Projection of a 6-
m Z-stack centered on the second intestinal segment is
shown. (A) Signals obtained using the 500-555-nm emission filter. (B) Signals obtained
using the 580-650-nm emission filter. (C) Merge of (A) and (B). Scale bar, 10
m
m
m.
acquire an additional image of the same field of view using the 488-nm laser and an
emission filter that collects signals at the 580-650-nm range. This allows the pref-
erential collection of autofluorescence with minimal GFP signals (
Figs. 3.2
A and
3.3
B). An overlay of (GFP
autofluorescence) and autofluorescence images allow
clear distinction of GFP signals from autofluorescence (
Fig. 3.3
C).
þ
3.4
VISUALIZATION OF LIPID DROPLETS BY TEM
3.4.1
Background
The defining feature of lipid droplets is the phospholipid monolayer that serves as a
delimiting membrane. This distinguishes lipid droplets from all other cytoplasmic or-
ganelles that are bound by membranes composed of phospholipid bilayers. TEM has
been used successfully to identify lipid droplets based on its phospholipid monolayer
membrane. A number of protocols are available for preparing
C. elegans
samples for
TEM (
Hall, Hartwieg, & Nguyen, 2012
). Depending on fixation and processing con-
ditions, lipid droplets in
C. elegans
can be electron lucent (
Leung et al., 1999
) or elec-
tron opaque (
Albert & Riddle, 1988
). We adopted a method that employed high
pressure freezing and freeze-substitution (
Hall et al., 2012; McDonald, 2007
), which
allowed the detection of the phospholipid monolayer around electron lucent lipid
droplet structures (
Zhang, Box, et al., 2010; Zhang, Trimble, et al., 2010
).
3.4.2
Methods
1.
Fixation is initiated by subjecting adult animals to high pressure freezing on a
Leica EM-PactI in a solution of 20% BSA in M9 buffer at
2050 bar.