Environmental Engineering Reference
In-Depth Information
Organic substrate
[
e
-
donor]
NH
+
or PO
3
-
4
4
SO
2-
4
H
2
S
NO
-
3
CH
4
Fe
2+
Mn
2+
O
2
Oxygen
reduction
Nitrate
reduction
Iron
reduction
Methanogenesis
Manganese
reduction
Sulfate
reduction
Time
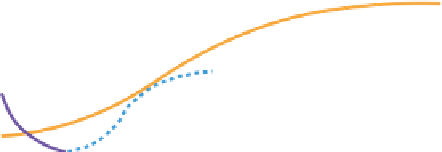
FIGURE A.2
Typical sequence of changes in biologically mediated redox reactions and concentrations of
redox-active materials that occur after a wetland soil is flooded and the organic material (long green line)
degrades. Solid lines show typical electron acceptors that are depleted as organic matter oxidation proceeds.
(
Modified from
Mitsch and Gosslink 1993
, after Reddy 2008
.)
observed either at a single point as electron acceptors are depleted over time, or mov-
ing through space away from well-oxygenated habitats, as in aquatic sediments or along
groundwater flow paths.
References
Fenchel, T., King, G.M., Blackburn, T.H., 1998. Bacterial biogeochemistry: The ecophysiology of mineral cycling,
second ed. Academic Press, San Diego, CA.
Maier, R.M., Pepper, I.L., Gerba, C.P., 2000. Environmental microbiology. Academic Press, San Diego, CA.
Mitsch, W.J., Gosselink, J.G., 1993. Wetlands, second ed. Van Nostrand Reinhold, New York, NY.
Reddy, K.R., DeLaune, R.D., 2008. Biogeochemistry of wetlands-science and applications. CRC Press, Boca
Raton, FL.
Stumm, W., Morgan, J.J., 1995. Aquatic chemistry: Chemical equilibria and rates in natural waters, third ed. Wiley,
New York, NY.
Wetzel, R.G., 2001. Limnology: Lake and river ecosystems, third ed. Academic Press, San Diego, CA.