Environmental Engineering Reference
In-Depth Information
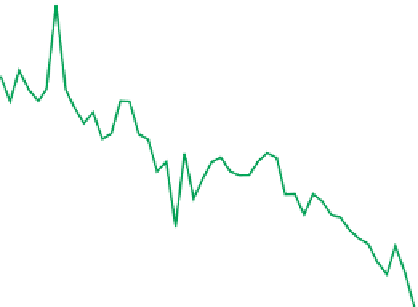
FIGURE 15.2
Annual,
volume-weighted hydrogen-ion
concentration in bulk precipita-
tion at the Hubbard Brook
Experimental Forest, NH. The
line represents a linear regres-
sion at p
PRECIPITATION TRENDS
100
90
80
,
0.05.
(Updated
70
from
Likens 2006
.
)
60
50
40
30
20
10
1960
1965
1970
1975
1980
1985
1990
1995
2000
2005
2010
2015
Water year
polluted (
Figure 15.2
). Studies of ice cores from glaciers in Greenland and elsewhere and
paleoecological analyses of lake sediments have also shown these historical changes in
precipitation chemistry.
In the early 1980s we began to study cloud- and fog-water chemistry, and found that it
could be especially polluted, even more so than rain at a specific site, and that acidic fog
events could be regional in extent (
Weathers et al. 1988
). One fog event collected near Bar
Harbor, ME, was black in color and had a pH of 2.42 (
Weathers et al. 1988
).
In addition to increasing emissions of SO
2
and NO
X
with growing industrialization after
World War II, another major factor affecting the regional pollution of the atmosphere and of
precipitation was that during the 1950s the heights of chimneys and smokestacks increased
dramatically (
Likens 1984, 1991
) carrying pollutants higher into the atmosphere, thereby
reducing local pollution at ground level, but enhancing the potential for long-distance
transport of pollutants in the atmosphere, affecting regions distant from the pollutant
source. Indeed, SO
2
and NO
X
can be transported in the atmosphere for thousands of
kilometers, allowing acid rain to cross political boundaries and impact ecosystems far
downwind from where these acid precursors are generated and emitted. This transfer of
pollutants through the atmosphere became highly charged politically, and ultimately led to
regulations controlling emissions in the United States (1990 Clean Air Act Amendments),
Canada, and Europe (see
Likens 2010
).
There are numerous ecological effects of acid rain on natural ecosystems, but they are
greatly complicated by diverse abiotic and biotic interactions and ecosystem impacts (e.g.,
Driscoll et al. 2001
). For example, acid rain can mobilize aluminum from the soil. When
aluminum is bound in minerals of the soil, it is harmless to biota, but in the dissolved
form it can be very toxic. Likewise, acid rain can leach calcium and magnesium from the
soil, thereby reducing the buffering capacity of the soil (
Likens et al. 1996, 1998; Likens