Environmental Engineering Reference
In-Depth Information
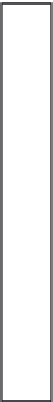
FIGURE 7.5
The N cycle in
the water column and sediment
layers of a thermally stratified
aquatic ecosystem such as a lake.
Uptake by primary producers
N fixation
Rapid recycling
Settling of organic matter
Mineralization
Immobilization
Nitrification
Deposition of organic matter
Aerobic mineralization, immobilization, nitrification
Denitrification
Sulfate reduction
Methanogenesis
Fermentation
to destruction of ozone in the stratosphere (where ozone absorbs UV radiation). The fac-
tors controlling the distribution of the gaseous end products of denitrification are complex
and variable, making relative assessment of the water quality benefits and atmospheric
chemistry problems associated with denitrification very difficult.
Other Dissimilatory Processes (DNRA and Anammox)
In addition to denitrification, there are other anaerobic processes where N-oxides serve
as electron acceptors to facilitate the dissimilation of energy. In the process of dissimilatory
nitrate reduction to ammonia (DNRA), NO
3
2
serves as an electron acceptor and NH
3
is the
end-product. This process is thought to be favored over denitrification in environments
where the ratio of energy sources to electron acceptors is high because DNRA consumes
more electrons than denitrification. Recent work has focused the ability of different energy
sources (e.g., reduced sulfur) to favor one pathway over the other.
In anaerobic oxidation of ammonia (anammox), the oxidation of ammonia as an
energy source is coupled to reduction of NO
2
2
as an electron acceptor, with N
2
as the
end-product. This process was originally discovered in sewage waste treatment plants, but