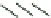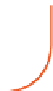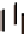Environmental Engineering Reference
In-Depth Information
Atmosphere
590
+161
0.2
Weathering
NPP and
respiration
60
59.6
Land
use
change
Land
sink
5.4
1.9
1.7
70.6
70
21.9
20
Vegetation
Soil and detritus
2300
+65 -124
Fossil fuels
3700
-220
0.4
0.8
Rivers
Surface ocean
Marine biota
3
5
0
39
900
+ 18
1.6
90.2
101
0.2
Weathering
11
Intermediate
and deep ocean
37,100
+ 100
0.2
Reservoir sizes in PgC
Fluxes and rates in PgC/yr
Surface sediment
150
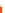
FIGURE 6.1
Simplified diagram of some of the key fluxes in the global carbon cycle. The values inside the
boxes are standing stocks (in Pg C); the arrows represent annual fluxes (Pg C/y). The black arrows and numbers
show the preindustrial values of standing stocks and fluxes; the red arrows and numbers indicate the changes
due to anthropogenic activity. Note there are some differences in the values shown on this figure and in
Table 6.1
due to different levels of aggregation and different time periods for the estimates. (The diagram is redrawn from
Climate Change (2007): The Physical Scientific Basis, Intergovernmental Panel on Climate Change.)
atmosphere, even the surface waters of most inland waters are usually greatly supersatu-
rated with respect to the atmosphere. A number of factors cause this disequilibrium,
including respiration in excess of photosynthesis, CO
2
inputs from ground water, or the
precipitation of CaCO
3
,whichreleasesCO
2
(see
Cole et al. 1994
).
Inorganic C is intimately involved in many of the acid-base reactions in soils and water.
Bicarbonate and carbonate constitute the major buffers in most natural waters and thus
account for most of the acid-neutralizing capacity (ANC; also called alkalinity). Free CO
2
is
the most dynamic of the constituents of DIC and is the dominant acid in most natural waters.
The ratio of CO
2
to HCO
3
2
and CO
3
5
is the major control of pH in most natural waters.
INORGANIC CARBON ON LAND AND SEDIMENTS
Solid-phase carbonate-bearing minerals on land are found principally in limestones and
related dolomite rocks, as well as some soils in arid regions (caliche;
Schlesinger 1982
).
Most of these terrestrial rocks are of marine origin. The amount of carbonate in rocks and