Biology Reference
In-Depth Information
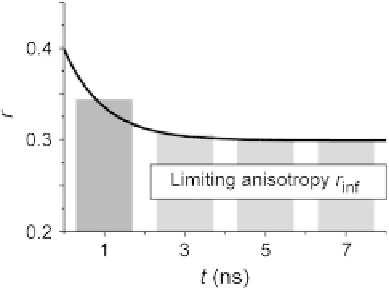
FIGURE 16.4
A schematic presentation of typical anisotropy decay due to homo-FRET. The limiting
anisotropy (rinf) is reached within a few nanoseconds and is dependent on the efficiency of
energy transfer.
Adapted from
Bader et al. (2007)
.
Importantly, the value of
r
inf
only depends on the cluster size of EGFR, while
r
ss
also
depends on the homo-FRET efficiency. Therefore, the clustering can be more accu-
rately determined, without the generally unknown contribution to the transfer effi-
ciency, by time-resolved anisotropy imaging. We determined
r
inf
values from
time-resolved measurements on cells expressing EGFR-FKBP-mGFP and EGFR-
2xFKBP-mGFP in the presence and absence of AP20187 (
Fig. 16.5
). This resulted
in relative changes in limiting anisotropy for dimers
r
/
r
inf
¼
0.82 and for oligomers
r
/
r
inf
¼
30% larger than in the steady-state
measurement. The difference is the result of the contribution from the initial part of
the anisotropy decay. This again indicates the advantage of time-resolved measure-
ments over steady-state measurements. Finally, we note that the measurement of the
anisotropy decays allows direct determination of the energy transfer rate
o
. In the ear-
lier examples, an energy transfer rate
o
of 70% can be estimated in the case of dimers.
Using the F¨rster distance
R
0
for homo energy transfer between twoGFPs of about 4.65
(
Sharma et al., 2004
), a distance of
0.72. These relative changes are almost
4 nm is found between two GFPs.
16.2
MATERIALS
16.2.1
Plasmid constructs
For homo-FRET measurements, a monomeric GFP (mGFP) was used as fluorophore
because normal GFP tends to cluster when expressed in high concentrations with a
K
D
of 110
m
M(
Zacharias, Violin, Newton, & Tsien, 2002
). mGFP has a point mu-
tation at position 206, resulting in a decrease in self-association and therefore more
reliable clustering measurements (
Zacharias et al., 2002
). This mGFP construct can