Biology Reference
In-Depth Information
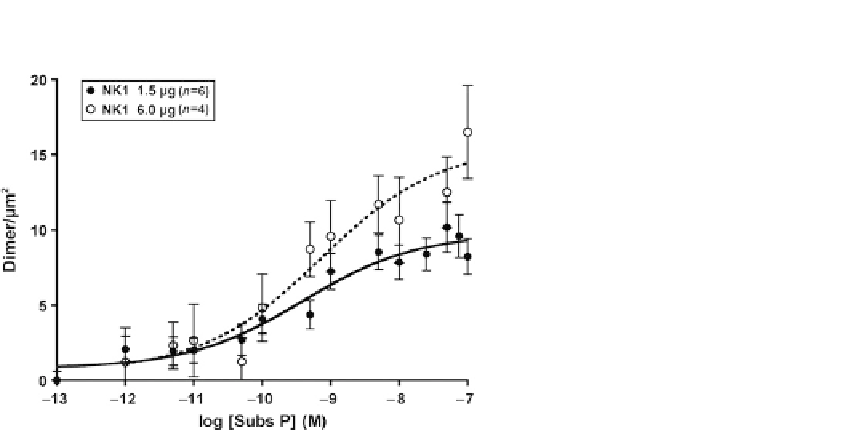
FIGURE 1.1
SpIDA allows for pharmacological characterization of EGFR transactivation by NK1 GPCR.
Dose-response curves of EGFR dimer density 1 min after substance P stimulation in CHO-k1
cells. Cells were transfected with either 1.5
m
g of GPCR DNA (n
¼
120 cells/point from six
individual experiments) or 6
60 cells/point from three individual experiments). Cells
were cotransfected with Rab5 S34N to prevent receptor internalization. Data are
means
m
g(n
¼
SEM.
resulting from the stimulation of a cotransfected GPCR, specifically stimulation
of neurokinin 1 receptors (NK-1R) by substance
P
. CHO-EGFR-eGFP cells
(
Brock, Hamelers, & Jovin, 1999
) were transiently transfected with NK-1R DNA,
and EGFR-eGFP dimerization was measured by SpIDA following agonist-specific
stimulation by increasing doses of substance
P
. Dimer densities were plotted using
the Hill-Langmuir binding isotherm model, and SpIDA results allowed us to extract
key parameters including
D
50
(ligand concentration required to obtain 50% maximum
dimer density signal) and
D
max
(maximal level of receptor dimer density signal).
The fitted parameters obtained,
D
50
and
D
max
, can be compared to similar pharma-
codynamic parameters like Bret50 and Fret50 commonlymeasured inRET-based phar-
macological studies (
Ayoub et al., 2007; Masri et al., 2008; Salahpour &Masri, 2007
).
Therefore, application of SpIDA to CLSM image analysis allows for the direct
comparison of transactivation efficiencies across different cell types and tissues. As
demonstrated in
Fig. 1.1
, SpIDA is able to reveal that the abundance of the GPCR
may be a limiting factor inRTK transactivation. Indeed, increasing the amount ofGPCR
cotransfected intocells results inan increased
D
max
, similar towhatwouldbeobserved in
thecaseofa
B
max
in a binding experiment (
Kenakin, 2009; Swift et al., 2011
).
The analysis further allows for quantification of receptor internalization, as being
evaluated by a reduction in surface receptor density. In the case of RTK transactiva-
tion by GPCRs, receptor internalization may be prevented by transfection of a rab5
mutant DNA construct, rab5 S34N (
Bucci et al., 1992; Li & Stahl, 1993
). Using a