Biomedical Engineering Reference
In-Depth Information
2.4 Use Case 2: Toxicity prediction on
compound collections
As a second, illustrative example, we look at the US FDA's Adverse Events
Reporting System database (AERS) [30]. This database is a unique source
of
in vivo
data on observations of the adverse outcomes of human
toxicities of drugs. Pharmatrope [31] has processed the AERS data
according to statistical considerations, and created the Titanium Adverse
Events Database and Models. For all compounds in the TCAMS we
predicted association with groups of human adverse events related to the
hepatobiliary tract and classifi ed the compounds' association with these
adverse events.
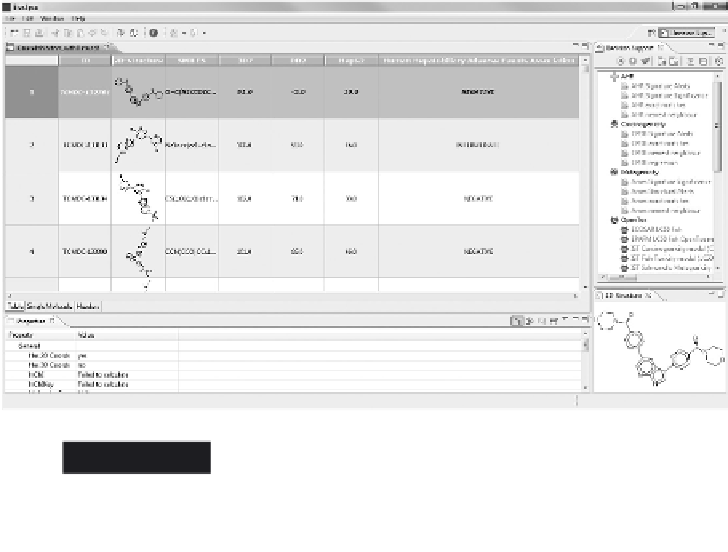
All data on the annotated kinase inhibitors in TCAMS - namely the
compounds' identifi ers and SMILES, the inhibition of
P. falciparum
3D7
and DD2, of human HepG2 cells, and the degree of association with
human adverse events - was combined into an SDF fi le and imported
into Bioclipse. Double-clicking the imported fi le in the Bioclipse
Navigator opens the table, which allows the rearrangement of the
columns (Figure 2.8).
Annotated kinase inhibitors of the TCAMS, imported
into Bioclipse as SDF together with data on the
association with human adverse events
Figure 2.8


Search WWH ::

Custom Search