Biology Reference
In-Depth Information
Blood Pressure Control
Fibrinolysis/Fibril
Formation
Angiotensin Converting
Enzyme
Complement
Fixation
ADAMTS
Proprotein
Processing
Procollagen C
Endopeptidase
MASP
Sperm-Egg
Fusion
Acrosin
Tissue Remodeling
MT-MMPs
Cell Surface
Proteolysis
Inflammation
Macrophage
Elastase
Cathepsin G
Alzheimer's Disease
Secretases
MMPs, meprins
TACE
Blood Clotting
Tumor Invasion and Metastasis
Thrombin
Leukocyte Elastase
MMPs, meprins, cathepsins
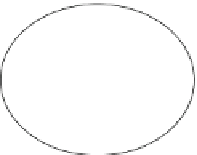
Fig. 4.1 Examples of proteolytic enzymes that act pericellularly and processes that they affect
such as bone morphogenetic protein-1 (BMP-1 or procollagen C-endopeptidase),
matrix metalloproteinase 1 (MMP-1, collagenase), blood coagulation proteinases
that act at a cell surface (thrombin), and complement proteases that form complexes
at cell surfaces. Mobile cells (e.g., leukocytes, cancer cells) often express mem-
brane-bound and secreted proteases that act pericellularly as they migrate and move
through epithelial barriers and the vasculature during extravagation and metastases
stages. In this chapter, we will mainly discuss meprin metalloproteinases. These
proteinases have membrane-bound and secreted forms and are highly expressed in
both polarized cells (kidney proximal tubule cells, and epithelial cells of the intestine
and skin) and leukocytes. The structure, function, and tissue expression of meprins
have been recently reviewed (Sterchi et al.
2008
), and thus these aspects will be only
briefly discussed in this chapter while advances in the last 2 years will be presented
more thoroughly.
4.2 Meprin Isoform Structure and Relation to Other Proteases
4.2.1 Discovery of Meprins
The meprins were discovered in mouse kidney and in human intestine in the early
1980s (Beynon et al.
1981
; Sterchi et al.
1982
). At that time, there were several
proteases known to be expressed in both these tissues, but the meprins had distinct