Biology Reference
In-Depth Information
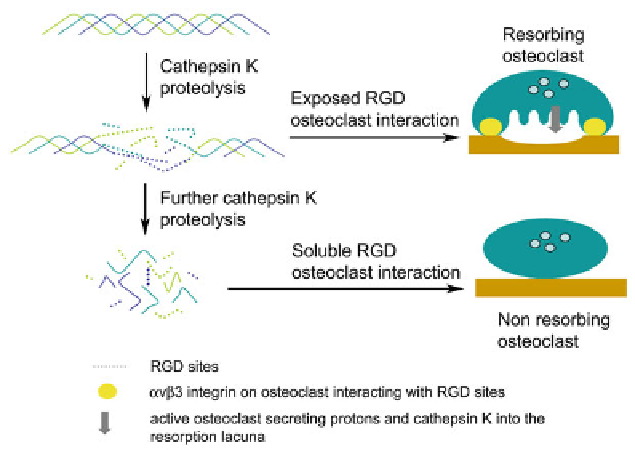
Fig. 2.5 Schematic representation of osteoclast regulation by cathepsin K-mediated RGD peptide
generation. Limited proteolysis of type I collagen by cathepsin K exposes cryptic RGD motifs,
which allows the cell to interact with the bone matrix and to obtain the bone resorbing status.
Further hydrolysis of bone collagen by cathepsin K leads to the release of soluble RGD motif
containing peptides which causes the disruption of the
a
v
b
3 integrin interaction with the bone
surface and subsequently to the inactivation of the osteoclast
Another site of cartilage digestion by cathepsin K-containing osteoclasts occurs
at the growth plate during endochondral ossification. During this process, the
cartilage scaffold laid down by chondrocytes is degraded by osteoclasts before
new bone can be deposited by osteoblasts. This process is essential for new long
bone formation. Malfunction of the matrix in certain pathological conditions such
as in mucopolysaccharidosis diseases and its effect on cathepsin K activity is
thought to contribute to the severe skeletal phenotype observed. MPS I is char-
acterized by an accumulation of heparan and dermatan sulfate in the matrix. Studies
have shown that these glycosaminoglycans not only colocalize with cathepsin K but
are also accompanied by decreased cathepsin K-mediated type II collagen digestion
(Wilson et al.
2009a
). The ability of excessive dermatan and heparan sulfates to
inhibit cathepsin K activity (Li et al.
2002
,
2004
) suggests that the activity of
cathepsin K during endochondral ossification is essential to long bone development.
2.5.2 Blood Vessels (Elastolytic and Collagenolytic Cathepsins)
The ECM of the blood vessel wall contains both elastin and collagen. In addition to
providing physical strength to the arteries, these proteins also act as a matrix for