Biology Reference
In-Depth Information
MMP-1
MMP-2
MMP-9
TIMP-2
(
α
4
β
1,
α
5
β
1)
(
α
v
β
5)
α
2
β
1
β
3
α
v
PAR-1
CD44
MT1-MMP
homodimer
α
2
β
1 integrin
α
v
β
3 integrin
β
1/
β
5 integrins
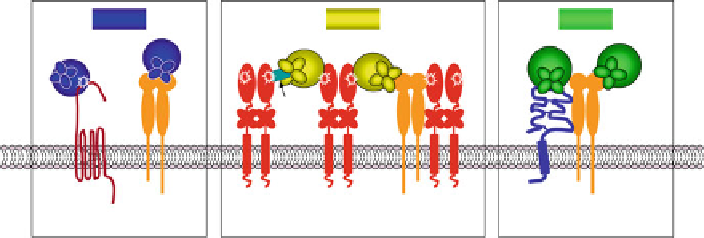
Fig. 7.2 Mechanisms of cell surface docking of secreted MMPs. The schematic depicts the
molecular mechanisms providing the means for cell surface docking of secreted MMPs and
focalizing soluble MMP activity to the pericellular space. Secreted MMPs can directly bind to
their cell surface target molecules, e.g., MMP-1 binds to PAR-1 to proteolytically activate the latent
receptor. Alternatively, secreted MMPs can be processed by the membrane-anchored MMPs, e.g.,
MMP-2 proenzyme is activated by cell surface MT1-MMP. Secreted MMPs can also dock via their
respective PEX domains to the corresponding cell surface molecules, e.g., MMP-1 binds to
a
2
b
1
integrin, MMP-2 binds to
a
v
b
3 integrin, and MMP-9 binds to CD44 and
b
1 and
b
5 integrin
subunits. A unique mechanism of cell surface docking is represented by MMP-2 activation that
requires binding of MMP-2 proenzyme to the C-terminal region of TIMP-2, which in turn is bound
via its N-terminal portion to the catalytic domain of MT1-MMP
PAR1-mediated signal transduction and inducing cell migration and invasion
in vitro and tumor growth in the orthotopic implantation in vivo (Boire et al.
2005
). Synergistic blocking of MMP-1 proteolytic activity and MMP-1/PAR-1
signaling in MDA-MB-231 xenograft tumors almost completely abrogates their
growth and, correspondingly, metastasis (Yang et al.
2009
). Both the collagenase
activity of MMP-1 and MMP-1-mediated PAR-1 signaling were demonstrated to be
necessary for matrix invasion and metastatic dissemination of melanoma cells
(Blackburn et al.
2009
). Secreted MMP-1 also binds to
1 integrin on keratino-
cytes, assisting simultaneous collagen-mediated migration and collagen cleavage at
the sites of
2
a
b
1-mediated adhesion (Dumin et al.
2001
).
The secretedMMP-2 proenzyme binds to
2
a
b
3 integrin (Brooks et al.
1998
), which
localizes MMP-2 to specific sites involved in cell adhesion but does not mediate the
proenzyme activation (Deryugina et al.
2001
). Instead, MMP-2 activation requires the
formation of proMMP-2/TIMP-2/MT1-MMP complex, in which TIMP-2-bound
proMMP-2 is activated by another TIMP-free MT1-MMP molecule (Strongin et al.
1993
,
1995
;Satoetal.
1994
). Since MT1-MMP binds and proteolytically activates
a
v
a
b
3 (Deryugina et al.
2002
;Ratnikovetal.
2002
), the formation of MMP-2/MT1-
MMP/
v
b
3 quaternary complexes pro-
vides a potent means for highly localized proteolysis at the sites of MMP-mediated
substrate degradation and
v
3 ternary or MMP-2/TIMP-2/MT1-MMP/
v
a
b
a
b
v
3-mediated cell adhesion and migration. In vivo, the
a
b
cooperation of
3 with MT1-MMP in MCF-7 breast carcinoma cells increased the
growth of orthotopic xenografts (Borrirukwanit et al.
2007
). SecretedMMP-2 can also
bind to the cell surface-tethered MT2-MMP, which activates the MMP-2 proenzyme
in a TIMP-2-independent manner (Morrison et al.
2001
).
v
a
b