Biology Reference
In-Depth Information
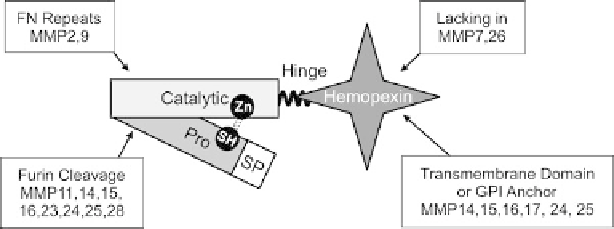
Fig. 1.1 Domain Structure of MMPs. The common motifs of MMPs are the pro- and catalytic
domains. The prodomain of a typical MMP is about 80 amino acids and contains the consensus
sequence PRCXXPD (except for MMP23). The catalytic domain contains three conserved
histidines in the sequence HEXXHXXGXXH, which ligate the active site Zn
2+
. Several MMPs
have a furin-recognition site within the C-terminal half of the prodomain allowing activation of
zymogen by proprotein convertases within the secretion pathway. As MMPs function in the
extracellular space, they each have a signal peptide (SP), the exception again being MMP23,
which has an N-terminal signal anchor. With the exceptions of MMP7, 23, and 26, MMPs have a
flexible proline-rich hinge region and a hemopexin-like C-terminal domain. Other additions
include transmembrane and cytosolic domains to the membrane-type MMPs, a glycosylpho-
sphatidylinositol (GPI) anchoring signal to MMP17 and 25, and gelatin-binding domains that
resemble similar motifs in fibronectin (FN) in MMP2 and 9
(MMP1, 3, 7, 8, 10, 12, 13, and 20) are clustered on chromosome 11 at 11q21-23.
Other MMP genes are scattered among chromosomes 1, 8, 12, 14, 16, 20, and 22
(Puente et al.
2003
).
1.2 Lexicon of Proteolysis
1.2.1 Breaking Down ECM
Three terms are often used when discussing proteolysis of ECM: turnover, degra-
dation, and remodeling. Although each term implies proteolysis, their meanings
within a broader biologic context are distinct. Thus, before we move on to discuss-
ing how MMPs function in matrix metabolism, we wish to define these terms and
how we will use them to convey distinct biologic processes.
Turnover
(or accretion)
refers to the normal physiologic replacement of protein (or any molecule) under
homeostatic conditions. For example, type IV collagen is deposited in a basement
membrane, and after some period of time, it is degraded and replaced with new type
IV collagen. Many proteins, particularly intracellular proteins such as transcription
factors and signaling factor, have relatively short half-lives, on the order of 48 h or
less. In contrast, many ECM proteins, especially large, insoluble polymers, such
as elastin and interstitial collagens, have extremely long half-lives, which has
led to the application of archeological methods to measure their turnover rate